Chemistry lecture 8 - SPRING 2015

LOGO
Lecture 8:
Solids and their properties
International University of Sarajevo
Course lecturer :
Jasmin Šutković
22 th April 2015
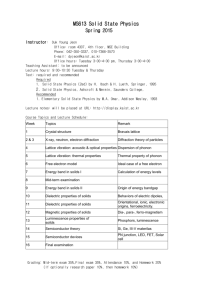
Contents
International University of Sarajevo
1. Solids – definition
2. Crystalline and Amorphous solids
3. X ray Diffraction
4. Defects in crystals
5. Bonding properties of solids
6. Bonding in metals and semiconductors
7. Superconductors
8. Polymeric solids
9. Contemporary materials
Solids – definition
The solid state is distinguished from the gas and liquid states by a rigid structure in which the atoms , ions or molecules re usually locked in space !
Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a gas does.
Solids , also called materials, are essential to today's technology !
Crystalline and
Amorphous Solids
• The particles that make up a solid material, whether ionic, molecular, covalent, or metallic, are held in place by strong attractive forces between them.
• In discussing solids, the positions of the atoms, molecules, or ions, which are fixed in place, are considered , rather than their motion.
• The constituents of a solid can be arranged in two general ways :
1. They can form a regular repeating three-dimensional (3D) structure called a crystal lattice , thus producing a crystalline solid.
2. They can aggregate with no particular order, in which they form an amorphous solid.
Crystalline VS. Amorphous solids
Crystalline solids(crystals)
Have distinctive internal structures that lead to distinctive flat surfaces, or faces
Each structure produces a distinctive pattern when exposed to X -rays that can be used to identify the material
There are three main types of crystalline solids: molecular, ionic and atomic.
Amorphous solids
When cleaved or broken, they produce fragments with irregular, often curved, surfaces.
They have poorly defined patterns when exposed to X -rays because their components are not arranged in a regular way.
An amorphous, translucent solid is called a glass.
Amorphous solids tend to soften slowly over a wide temperature range rather than having a well-defined melting point like a crystalline solid.
Amorphous solids cont…
Amorphous materials are engineered systems.
For example :Thin film lubricants, and bulk metallic glasses are seemingly disparate systems which are similar in that they possess an amorphous structure.
Colloids, emulsions, window glass, dense polymers, and even biological tissues are other examples.
Examples of amorphous materials
X-Ray Diffraction
• X -rays are a useful tool for obtaining information about the structures of crystalline substances because the wavelength of
X-ray radiation
• In X -ray diffraction, a beam of X -rays is aimed at a sample of a crystalline material, and the X -rays are diffracted by layers of atoms in the crystalline lattice.
• When the beam strikes photographic film, it produces an X -ray diffraction pattern, which consists of dark spots on a light background.
Defects in Crystals
The ideal crystal has an infinite 3D repetition of identical units, which may be atoms or molecules.
Real crystals contain large numbers of defects
(typically more than 10 4 per milligram) ranging from variable amounts of impurities to missing or misplaced atoms or ions
Reasons for defects
Defects occur for three main reasons:
1. It is impossible to obtain any substance in 100% pure form; some impurities are always present.
2. Forming a crystal requires cooling the liquid phase to allow all atoms, ions, or molecules to find their proper positions, but cooling results in one or more components being trapped in the wrong place in a lattice or in areas where two lattices that grow separately intersect.
3. Applying an external stress to a crystal can cause microscopic regions of the lattice to move with respect with the rest; this results in imperfect alignment.
Defects in Metals
Types of defects :
A point defect is any defect that involves only a single particle
(a lattice point) or a very small set of points.
• Vacancies , Interstitials and Impurities
A line defect is restricted to a row of lattice points.
• Edge dislocations, and Screw dislocations.
A planer defect involves an entire plane of lattice points in a crystal.
Vacancies
A Vacancy is the absence of an atom from a site normally occupied in the lattice.
An empty lattice site is a vacancy
Interstitials
An Interstitial is an atom on a non-lattice site.
There needs to be enough room for it, so this type of defect occurs in open covalent structures, or metallic structures with large atoms.
An interstitial is an off lattice atom which may be a foreign atom or a regular atom
An interstitial may form by an atom moving to an off lattice site and create a vacancy at the same time
Impurities
An Impurity is the substitution of a regular lattice atom with an atom that does not normally occupy that site.
The atom may come from within the crystal, (e.g. a Chlorine atom on a Sodium site in a NaCl crystal) or from the addition of impurities.
A foreign atom or a regular atom out of place, is an impurity.
Line effect – dislocation
An Edge dislocation in a Metal may be regarded as the insertion (or removal) of an extra half plane of atoms in the crystal structure .
Memory Metal
The compound NiTi (Nickel Titanium) is known as memory metal , and it illustrates the importance of deformations.
• If a sample of this metal is warmed from room temperature to a temperature higher than 50ºC, it will revert to a shape in which it has been previously set.
Example : Flexon is a fatigue-resistant alloy of Ti and Ni that is used as a frame for glasses because of its durability and corrosion resistance.
Correlation between Bonding and the Properties of Solids
Based on the nature of the forces that hold the component atoms, molecules, or ions together, solids are classified as
1. ionic
2. molecular
3. covalent
4. metallic
Variation in the relative strengths of these four types of interactions correlates with their wide variation in properties of these four kinds of solids.
Ionic Solids
– Consist of positively and negatively charged ions held together by electrostatic forces
– Strength of the attractive forces depends on the charge and size of the ions that make up the lattice and determines many of the physical properties of the crystal
– Lattice energy , the energy required to separate 1 mol of the crystalline ionic solid into its component ions in the gas phase , is directly proportional to the product of the ionic charges and inversely proportional to the sum of the sizes of the ions
Characteristics :
– Poor conductors of heat and electricity
– High melting points
– Hard but brittle; shatter under stress
– Dense with a dull surface
NaCl
Molecular Solids
– Consist of atoms or molecules held together by dipole-dipole interactions, London dispersion forces, or hydrogen bonds
– Intermolecular interactions in a molecular solid are relatively weak compared with ionic and covalent bonds
– Tend to be soft, low melting, and easily vaporized
Characteristics :
– Poor conductors of heat and electricity
– Low density
– Dull surface
Covalent Solids
– Formed by networks or chains of atoms or molecules held together by covalent bonds
– A perfect single crystal of a covalent solid is a single giant molecule
– Tend to be very hard and have high melting points
– Not easily deformed
– Brittle, tend to shatter when subjected to large stresses
– Poor conductors of heat and electricity
– Low density
Metallic Solids
– Packing efficiency in metallic crystals tends to be high, so metallic solids are dense, with each atom having as many as
12 nearest neighbors
– Every lattice point in a pure metallic element is occupied by an atom of the same metal
– Have high electrical and thermal conductivity
– Have high heat capacity
– Are malleable and ductile
– Easily deformed under stress
– High density
– Melting points depend strongly on electron configuration
Metallic Solids cont..
– Bonding in metallic solids is quite different from the bonding in the other kinds of solids; the valence electrons are delocalized throughout the crystal , providing a strong cohesive force that holds the metal atoms together
– Strength of metallic bonds varies dramatically!
– Metallic bonds tend to be weakest for elements that have nearly empty or nearly full valence shells , and are strongest for elements with half-filled valence shells
Band Theory of solids
Band theory explains the correlation between the valence electron configuration of a metal and the strength of metallic bonding.
The spacing between energy levels is so small in metals that the levels essentially merge into a band.
When the band is occupied by valence electrons, it is called a valence band!
A partially filled or low lying empty band of energy levels, which is required for electrical conductivity, is a conduction band.
Band theory provides a good explanation of metallic luster and metallic colors.
http://chemwiki.ucdavis.edu/u_Materials/Electronic_Properties/Band_T heory_of_Metals_and_Insultators
Insulators
• Electrical insulators are materials that conduct electricity poorly because their valence bands are full.
.
Semiconductors
• A semiconductor is a material which has electrical conductivity between that of a conductor such as copper and an insulator such as glass. The conductivity of a semiconductor increases with increasing temperature, behavior opposite to that of a metal!
You find semiconductors at the heart of microprocessor chips as well as transistors. Anything that's computerized or uses radio waves depends on semiconductors.
SEMICONDUCTORs
Diodes
Semiconductor elements in PSE
Superconductor cont …
Superconductors
Superconductivity is the total disappearance of electrical resistance below a definite temperature called the transition temperature (T c
)
At temperatures lower than their T c
, superconductors completely expel a magnetic field from their interior, a phenomenon called the Meissner effect.
Example
In magnetic levitation , a small magnet “floats” over a disk of a high-temperature superconducting material (YBa
2
Cu
3
O
7− x
) cooled in liquid nitrogen.
The superconductor needs to be at a temperature below
-184 degrees Celsius
If the scientist succeed to make it work at room temperature then we may see something like this ….
BCS Theory
• Bardeen, Cooper, and Schrieffer formulated a theory for superconductivity called the BCS theory.
• According to the BCS theory, electrons are able to travel through a solid with zero resistance because of an attractive interaction between two electrons that are at some distance from each other.
Polymeric solids
Many of the molecular materials in consumer goods today, however, have very high molecular masses, ranging from thousands to millions of atomic mass units, and are formed from a carefully controlled series of reactions that produce giant molecules called polymers (from the Greek poly and meros , meaning “many parts”).
Polymers are used in corrective eye lenses, plastic containers, clothing and textiles, and medical implant devices, among many other uses.
They consist of basic structural units called monomers, which are repeated many times in each molecule.
Polymerization is the process by which monomers are connected into chains or networks by covalent bonds
Polymer and Plastic
Many people confuse the terms plastics and polymers .
Plastic is the property of a material that allows it to be molded into almost any shape.
Although many plastics are polymers, many polymers are not plastics.
Biological Polymers:
Peptides and Proteins
• Biological polymers are crucial components of all organisms and form the fabric of our lives.
Monomers of many biological polymers are the amino acids ,which are linked together by amide bonds (peptide bonds).
Chains that contain fewer than about 50 amino acid residues are called
peptides.
Chains that contain more than 50 amino acid residues are called proteins .
Many proteins are enzymes , catalysts that increase the rate of a biological reaction.
Structural formula and the peptide bond
The structural formula for a peptide or protein is written with the free amino group on the left ( N-terminus ) and the free carboxylate group on the right (C-terminus).
Synthetic Polymers
• Synthetic polymers include plastics, fibers, and rubbers.
• Fibers are particles that are more than a hundred times longer than they are wide.
• The best-known example of a synthetic polymer is nylon , whose monomers are linked by amide bonds, so its physical properties are similar to those of some proteins.
• Replacing the flexible CH
2 units in nylon by aromatic rings produces a stiffer and stronger polymer called Kevlar .
Synthetic Polymers cont.…
• Polyesters are synthetic polymers that are linked by ester bonds.
• Substances such as glass can also be formed into fibers, producing fiberglass.
• Pyrolysis is a high-temperature decomposition reaction that can form fibers.
Polyester examples
Fiberglass material
Contemporary Materials
• In addition to polymers, other materials such as ceramics, high-strength alloys, and composites play a major role in almost every aspect of our lives.
Ceramic High Strength alloys Composites
Quiz II, 8
th
May
Prepare Lecture 6,7 and 8!
Quiz II will start at 14h and finish at 15h