ME 152 Thermodynamics
advertisement
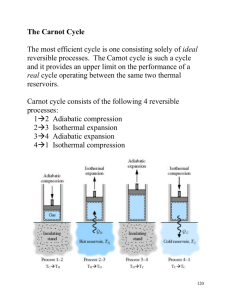
The Second Law of Thermodynamics Cengel & Boles, Chapter 5 ME 152 1 The Second Law of Thermodynamics • So far we have studied: – conservation of energy (i.e., First Law of Thermodynamics) – conservation of mass – tabulated thermodynamic properties and equations of state (e.g., ideal gas law) • There is a need for another law – one that tells us what sort of processes are possible while satisfying conservation principles ME 152 2 Second Law Statements • Like the 1st Law, the 2nd Law of Thermodynamics is based upon a long history of scientific experimentation • There is no single verbal or math statement for this Law - instead, there is a collection of statements, deductions, and corollaries regarding thermodynamic processes that together form the 2nd Law • Two popular statements: – Clausius statement – Kelvin-Planck statement ME 152 3 Kelvin-Planck Statement • “It is impossible for any device that operates as a cycle to receive heat from a single thermal reservoir and produce an equivalent amount of work” ME 152 4 Clausius Statement • “It is impossible to construct a device that operates as a cycle whose sole effect is the transfer of heat from a lower temperature reservoir to a higher temperature reservoir” ME 152 5 Thermodynamic Cycles • Cycle energy balance Qcycle Wcycle Ecycle Qcycle Wcycle • Types of cycles – heat engines, (aka power cycles) – refrigeration and heat pump cycles ME 152 6 Heat Engines • Net (cycle) work output: Wcycle Qcycle or Wnet ,out Qin Qout QH QL • Thermal efficiency desired output Wnet ,out th required input QH QH QL QL 1 QH QH ME 152 7 Refrigeration & Heat Pump Cycles • Net work input: Wnet ,in Qin Qout or Wnet ,in QH QL • Coefficient of performance (COP) desired output required input Q QL COPR L Wnet ,in QH QL COP QH QH COPHP Wnet ,in QH QL ME 152 (refrigera tor) (heat pump) 8 Reversible Processes • Reversible Process: a process that can be reversed, allowing system and surroundings to be restored to their initial states – no heat transfer – no net work – e.g., adiabatic compression/expansion of a gas in a frictionless piston device: ME 152 9 Reversible Processes, cont. • Reversible processes are considered ideal processes – no energy is “wasted”, i.e., all energy can be recovered or restored – they can produce the maximum amount of work (e.g., in a turbine) – they can consume the least amount of work (e.g., in a compressor or pump) – they can produce the maximum KE increase (e.g., in a nozzle) – when configured as a cycle, they produce the maximum performance (i.e., the highest th or COP) ME 152 10 Irreversible Processes • Irreversible Process - process that does not allow system and surroundings to be restored to initial state – such a process contains “irreversibilities” – all real processes have irreversibilities – examples: • • • • • • • • heat transfer through a temperature difference unrestrained expansion of a fluid spontaneous chemical reaction spontaneous mixing of different fluids sliding friction or viscous fluid flow electric current through a resistance magnetization with hysteresis inelastic deformation ME 152 11 Internally Reversible Processes • A process is called internally reversible if no irreversibilities occur within the boundary of the system – the system can be restored to its initial state but not the surroundings – comparable to concept of a point mass, frictionless pulley, rigid beam, etc. – allows one to determine best theoretical performance of a system, then apply efficiencies or correction factors to obtain actual performance ME 152 12 Externally Reversible Processes • A process is called externally reversible if no irreversibilities occur outside the boundary of the system – heat transfer between a reservoir and a system is an externally reversible process if the outer surface of the system is at the reservoir temperature ME 152 13 The Carnot Principles • Several corollaries (the Carnot principles) can be deduced from the Kelvin-Planck statement: – the thermal efficiency of any heat engine must be less than 100% th Wnet ,out QH 1 QL QH – th of an irreversible heat engine is always less than that of a reversible heat engine – all reversible heat engines operating between the same two thermal reservoirs must have the same th ME 152 14 The Kelvin Temperature Scale • Consider a reversible heat engine operating between TH and TL : th,rev QL f1 (TL , TH ) 1 QH QL QH 1 f1 (TL , TH ) rev f 2 (TL , TH ) • Kelvin proposed a simple relation: QL T L QH rev TH ME 152 15 The Kelvin Temperature Scale, cont. • Kelvin’s choice equates the ratio of heat transfers in a reversible heat engine to a the ratio of absolute temperatures • Need a reference to define the magnitude of a kelvin (1 K) - the triple point of water is assigned 273.16 K: QH TH 273.16 Q L,tp rev ME 152 16 Maximum Performance of Cycles • Carnot Heat Engine: th,rev 1 TL TH • Carnot Refrigerator: COPR ,rev TL 1 TH TL TH / TL 1 • Carnot Heat Pump: COPHP,rev TH 1 TH TL 1 TL / TH ME 152 17 The Carnot Cycle • The Carnot cycle is the best-known reversible cycle, consisting of four reversible processes: – adiabatic compression from temperature TL to TH – isothermal expansion with heat input QH from reservoir at TH – adiabatic expansion from temperature TH to TL – isothermal compression with heat rejection QL to reservoir at TL ME 152 18 The Carnot Cycle, cont. • Note: – the heat transfers (QH , QL) can only be reversible if no temperature difference exists between the reservoir and system (working fluid) – the processes described constitute a power cycle; it produces net work and operates clockwise on a P-v diagram – The Carnot heat engine can be reversed (operating counter-clockwise on a P-v diagram) to become a Carnot refrigerator or heat pump – the thermal efficiency and coefficients of performance of Carnot cycles correspond to maximum performance ME 152 19





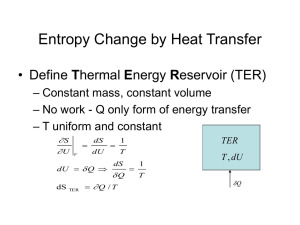
![Introduction to Second Law (contd.) [Lecture 5].](http://s2.studylib.net/store/data/005616309_1-e04677ea698eaf2815262e3c7bbb995c-300x300.png)