Introduction to Second Law (contd.) [Lecture 5].
advertisement
![Introduction to Second Law (contd.) [Lecture 5].](http://s2.studylib.net/store/data/005616309_1-e04677ea698eaf2815262e3c7bbb995c-768x994.png)
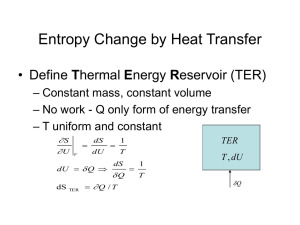
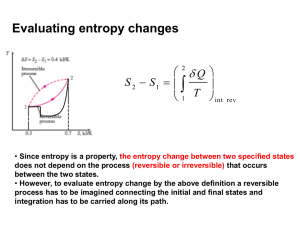
The equivalence of the ideal gas and thermodynamic temperature scales Choose the ideal gas as the working fluid for a Carnot engine or refrigerator and place one of the reservoir in thermal equilibrium with water at triple point. V3 V4 V2 V1 tideal g as tideal g as ,tp 1 1 Here, Q Qtp rev V2 Rtideal gas ln tideal gas V1 T V3 273.16 273.16 Rtideal gas,tp ln V4 Q(t ) T (t ) 273.16 Qtp Conclusion: T(tideal gas)=tideal gas rev (the two scales are identical) tideal gas 273.16 p ptp The maximum efficiency of a cyclic heat engine Q W QH QL 1 L QH QH QH From the Carnot principles: rev Max QL TL QH rev TH rev R e v Max TL 1 TH TL 1 TH T1 Q1 The Kelvin Planck statement for a cycle with contact with one reservoir W Q1 0 W C • Sign convention: heat flow into system (cyclic device C) is positive . work done by system is positive T1 Q1 Using Carnot corollaries, the Kelvin-Planck statement for one reservoir can be extended to two reservoirs Q1 Q2 0 T1 T2 C Q2 T2 Second Law for cyclic devices in contact with two reservoirs • Sign convention: heat flow into the system (cyclic device C) is positive. • C can be either engine/refrigerator • No arrow for work is shown in figure (to save space). T1 Q1 The second law for a cyclic device exchanging heat with one/two reservoirs Qj N C Q2 T2 T j 1 0 for N=1,2 j Note : T (thermodynamic temperature in Kelvin scale) is always non-negative Is this also true for an integer N larger than 2? Procedure for extending the second law N reservoirs To prove that the statement N Qj T j 1 0 i is true for all positive integers N Proof by induction. Steps: • Assume when any closed system undergoes a cyclic process, the statement is true for all N ≥ 2 . • Show that if the statement for N is true, then the statement is also true for N+1. • Since, we already know the statement is true for N=2, the step above means it is true for N=3, which again means the statement is true for N=4 and so on for all positive integers N. Show that if the statement for N is true, then the statement is also true for N+1. N T1 Qj T j 1 0 for system C j Choose Q’N+1 such that (N+1)th reservoir has no net heat exchange Q1 TN+1 QN+1 C QN 1 QN 1 0 2nd Law for reversible cycle R QN 1 Q 'N 0 TN 1 TN For R+C system (blue curve): ' N 1 j N N Q Q TN j 1 T j Q R Q’N QN 0 TN j 1 T j N Qj Show that if the statement for N is true, then the statement is also true for N+1. N T1 Qj T j 1 0 for system C j Choose Q’N+1 such that (N+1)th reservoir has no net heat exchange Q1 TN+1 QN+1 C QN 1 QN 1 0 2nd Law for reversible cycle R QN 1 Q 'N 0 TN 1 TN For R+C system (blue curve): R Q’N QN 0 TN j 1 T j N Qj QN 0 TN j 1 T j N T1 T2 Q Q2 1 Qj Using and QN 1 Q 'N 0 TN 1 TN Tj Qj R+C QN 1 QN 1 0 QN QN 1 TN TN 1 Q Q’ N N QN TN QN 1 N 1 Q j 0 TN 1 j 1 Tj j 1 T j N Qj Method 2: Idea is to use the KP statement for one reservoir; This requires Q '1 introducing construction to make each R1 existing reservoir redundant by introducing suitable reversible Q01 engines connected to a common reservoir. Since system C+R1+R2+..Rj+..RN is exchanging heat with one reservoir, KP statementQ0≤0 Render each reservoir Tj Idle by choosing T1 Qj Qj 0 For each reversible cycle Rj Q0 j Q1 T0 Q '2 C R2 Q 'j Rj Q 'N RN T0 Q0 N Q0 j Qj 0 Tj Q0 j T0 T0 Qj Tj Qj Tj Q02 N N Qj j 1 j 1 Tj Q0 Q0 j T0 N Qj j 1 Tj 0 The Clausius inequality ˜ Q T 0 • T is the temperature of boundary through which heat transfer Q occurs. • The integral with a circle is performed over a cycle and all parts of system boundary that have different temperatures. The circle is used to denote a cyclic process. • Valid for both irreversible and reversible cycles. • Actually is a way of stating second law for cycles. Q System Some conclusions from Clausius inequality Q 0 T Q 0 T Q 0 T for an irreversible cycles for an internally reversible cycles (also known as Clausius theorem) is impossible Definition of a new property: entropy • Regardless of how the reversible cycle is executed following integral over a cycle is zero. Q T int rev 0 Definition of a new property: entropy • • Regardless of how the reversible cycle is executed the integral over a cycle is zero. Q T int rev 0 The integrand must be signifying the differential of a property Q dS T int rev Definition of a new property: entropy • Regardless of how the reversible cycle is executed the integral over a cycle is zero. • The integrand must be signifying the differential of a property • ˜ Entropy change in a process: Q 0 T int rev Q dS T int rev 2 Q S 2 S1 1 T int rev Note: We now have a definition for entropy change and not for the absolute value of entropy. So when you see only S anywhere, understand S to be an entropy change calculated from some reference state (S-Sref).