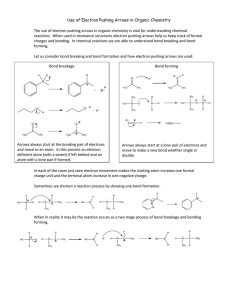
Introduction to Bonding
advertisement
Octet Exceptions & VSEPR Model March 5, 2007 More Octet Exceptions • Some molecules have too many electrons to hold to the octet rules. F F • SF6 is an example F F F F S S F F F F F F • Only elements in period 3 and higher can exceed the octet rule. • If there is a question as to where the extra electrons go, put them on the central atom. Cl Xe Cl • XeCl2 VSEPR Model • • 1. 2. 3. 4. Predicting the 3-D molecular structure of an atom. The model attempts to minimize electron-pair repulsions. Begin with the Lewis Structure! Predict the arrangement of the electron pairs to minimize repulsions. Determine the positions of the bonded atoms. Determine the molecular structure name. 4 Electron Groups • Tetrahedral arrangement • NOT 90º • 109.5º angle 4 Electron Groups (1 lone pair) • NH3 (ammonia) 4 Electron Groups (2 Lone Pairs) • H2O (water) Lone Pairs and Bond Angles • The more lone pairs, the smaller the bond angle. Exam Practice • Which of the following molecules or ions has the smallest bond angle? or the largest? smallest • a. H2O largest • b. HCN • c. O3 • d. CH4 • e. NO3 5 Electron Groups • PCl5 – trigonal bipyramid • bond angles of 90, 120 (and 180) http://www.elmhurst.edu/~chm/vchembook/220trigbipy.html 6 Electron Groups