Cross word review BLANK
advertisement

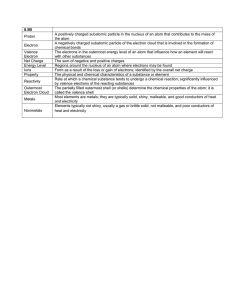
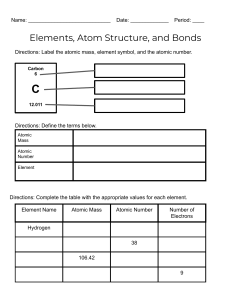
9 Physical Science Semester Review Across 5. A graduated cylinder with more lines has a higher ___ 6. Chlorine has ___ valence electrons 9. Highly reactive nonmetal family 11. The strong ___ force holds the nucleus of the atom together 12. Heat transfer that possess circular motions like those producing wind 13. Thermal energy is a type of ___ energy 14. Sand, iron, aluminum mixture is a ___ mixture 19. This scientist discovered the positive charged nucleus 23. A type of bond between a cation and an anion 25. This state of matter has the fastest moving molecules 26. A charged atom 27. The elements that posses traits of both metals and nonmetals 28. Anything that has mass and volume 30. Melting ice is an example of a ___ change 33. This number tells you the # of protons 34. Opposite charges ___ each other 35. Plastic, wood and Styrofoam are examples 36. The flow of thermal energy 37. This subatomic particle has a positive charge and gives the element its identity 39. This variable is typically found on the X axis 42. The least reactive family of elements 45. Carbon-12 and Carbon-14 are ___ of each other 49. These are found on the right side of a chemical equation 51. When oil is poured into a beaker of water it settles on top of the water. The oil must be ___ dense. 52. When a solid goes directly to a gas 53. The ability to be pounded into thin sheets 54. This state of matter makes the best conductor Down 1. The overall charge of the nucleus 2. This type of bond occurs when carbon bonds with oxygen 3. Melting point and ___ point of water occurs at 0°C 4. The type of heat transfer that requires direct contact 7. Electrons in the outer energy level are called 8. "Sea of electrons" creates this type of bond 10. This element has 16 protons 15. Nonmetals are found on the ___ side of the periodic table 16. Heat always flows from ___ to ___ (write answers as one word) 17. The ___ number of lithium is 1+ 18. Burning a piece of paper is an example of a ___ change 20. Feeling the sunlight on your face demonstrates this type of heat transfer 21. The ___ number - Atomic number = # of neutrons 22. When naming ionic bonds with transition metals, ___ numerals are used to show the oxidation number 24. When classifying matter, pure substances are broken down into elements and ___ 29. This subatomic particle is found in energy levels 31. The resistance to being scratched 32. ___ of mass 38. Na-23 has 12 ___ (which subatomic particle) 40. A type of reaction that releases energy and therefore feels warm 41. Water ___ can be used to determine the volume of an object 43. The ___ number tells you the # of valence electrons an atom possesses 44. A neutral atom of oxygen will have ___ electrons 46. During a ___ change the temperature of the liquid stays the same 47. When electrons gain energy they move from the ground state to the ___ state 48. Family name for Group 1 elements is __ metals 50. Mass / volume = ___ 9 Physical Science Semester Review