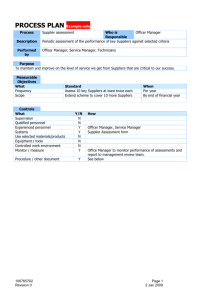
Supplier Corrective Action Guide
advertisement

GKN St. Louis Guide to Corrective Action Response Development GKN Supplier Quality Engineer 1 1. Complete Supplier Company Name, Supplier Address, and Supplier Vendor Code (GKN internal code) 2. Complete GKN Supplier Quality Engineer, Phone Number, e-mail Address, and FAX Number 3. Complete Supplier Contact Telephone Number, FAX Number, and e-mail Address 4. Complete CAR No. and CAR Revision Letter 5. Complete GKN Buyer, Phone Number, and e-mail address 6. Non-Conformance Description: A detailed description of the nonconformance. Do not refer to other documents in this section. Provide complete information, including part number and quantity affected. 7. Issue Date: The date the GKN SQE issued the CAR to the supplier. This date will drive the response dates for the following sections. 3 4 2 5 6 7 Supplier 8. This form was completed by: Enter the name of the individual responsible for completing the form and submitting it to GKN. 9. Containment Action Taken Pending Corrective Action Implementation: Describe the actions taken to stop or contain the nonconformance; purge actions, stop shipments, re-inspection of stock, etc. Who is responsible for completion of the actions: Provide names and titles of the person(s) responsible for the containment actions. What are the timelines for the actions, including completion: Provide milestone dates for the containments. If multiple tasks are required, list them individually, not just the final completion date of all the actions. 10. Immediate Action Taken to Correct the Specific Nonconformance: Provide information on what is being done to correct the specific nonconformance; revising programming, providing additional training, creating visual aids, etc. Who is responsible for the completion of the actions: Provide names and titles of the person(s) responsible for correcting the nonconformance. What are the timelines for the actions, including completion date: Provide milestone dates for correcting the specific nonconformance. If multiple tasks are required, list them individually, not just the final completion date of all the actions. 8 9 10 Information up to this point must be returned to the GKN SQE within 3 Business Days of Issue Date. Supplier 11. Root Cause of the Nonconformance: In this section a 5-Why analysis will be implemented, in order to determine the true root cause of the nonconformance. 12. Basic Cause: 1-Why: Provide the most basic cause of the action, without restating the nonconformance statement (Answer “Why is this part nonconforming?”) 13. 2 – 5 Why: Once the answer to Why 1 has been determined, ask “Why?” again to that answer. Continue this process until all 5 Whys are completed. By asking “Why?” 5 times, you can dig into a problem deeply enough to understand the ultimate root cause. 11 12 14. Root Cause Statement: Enter the root cause of the problem determined through the 5-Why analysis. This is not a restatement of the 5th Why. 13 14 This section must be completed and returned to GKN SQE within 14 days of Issue Date (or return of part). Supplier 15 16 17 15. Permanent Action Taken to Prevent Recurrence: Provide information on actions are being taken to prevent the identified root cause from recurring. Active / Passive Mistake Proof: Check which box applies to the actions being taken. An Active Mistake Proof would be a physical device or work element that prevents recurrence of a mistake. Active mistake proofing disables the mistake from being made again. A Passive Mistake Proof would be an advisory or cautionary intervention on how to perform an action but lacks the physical device or element used in an active mistake proofing. MISTAKE CAN STILL OCCUR. Who is responsible for completion of the actions: Provide the names and titles of the person(s) responsible for preventing recurrence. What are the timelines for the actions, including completion dates: Identify the milestone dates correcting the root cause. If multiple tasks are required, list them individually, not just the final completion date of all the actions. 16. Corrective Action Verification Plan: Provide a plan to verify the effectiveness of corrective actions. Detail the verification actions that will be undertaken to ensure what was proposed was completed, and it was effective in curing the nonconformance. These verifications must be proven . Statements such as “all actions complete” is not sufficient proof of effective verification. Who is responsible for the completion of the actions: Provide the names and titles of the person(s) responsible for verifying the specific nonconformance has been corrected. What are the timelines for the actions, including completion: Identify the milestone dates for verification actions. If multiple tasks are required, list them individually, not just the final completion date of all the actions. 17. Actions taken to Identify and Correct Other Products / Processes Similarly Effected: List the similar issues you have examined to find out if there are other process that may be affected. This section must be completed and returned to GKN SQE within 14 days of Issue Date (or return of part). Root Cause and Corrective Action Four basic parts of process 1.Identify and Describe the problem (Plan) 2.Investigate/determine key causes/Select solution (Organize) 3.Verify Implementation (Implement) 4.Monitor and re-validate (Monitor) Remember…at each step, you need to identify who is responsible for the action and the timeline of when is the actions are to be completed. Define the Problem Problems can be ‘systemic’ or ‘isolated’ in occurrence Systemic Problems Can be attributed to process design or product design Will continue to occur at the same or varying degree of severity if not corrected Isolated Problems Caused by problems with compliance, standards, or efficiency Un-natural occurrence to a process or system Problem statement should contain information on what limits are acceptable and how the limits were exceeded. It should be specific to one issue. If more than one problem is identified, each should be addressed individually to ensure an accurate solution is reached. Investigate the Problem Collect Data Investigate all areas of product flow; don’t overlook 2nd tier suppliers Check internal nonconformance databases for similar anomalies – if any are found review the countermeasures implemented and determine why they didn’t work Review personnel training records to determine if this is a contributing factor Review tooling to verify correct configuration and revision level Talk to people linked to process; recreate situation where problem occurred Determine Root Cause by performing 5 Why Analysis Root Cause Determination Root Cause is the basic reason for an anomaly which, when eliminated, will prevent the problem from recurring. Avoid general classifications like operator error or equipment failure Should be specific enough to generate specific corrective and preventive actions There are three methods used to determine Root Cause 5 Why Chain Method Fish bone or Multiple Cause Analysis Determining Root Cause will result in a robust countermeasure and corrective action that will prevent a recurrence Example of 5-Why Root Cause Investigation Problem: Why? Machine failed to operate when switch turned on Why did the machine fail? The motor burned out Why? Why did the motor burn out? The shaft seized Why? Why did the shaft seize? There was no lubrication Why? Why was there no lubrication? The filter was clogged Why? Root Cause: Why was the filter clogged? Wrong filter was installed by maintenance Wrong filter installed Temporary CM: Motor replaced and correct filter installed; effective 3/3/03 Permanent CM: All filters color coded by size; color indicator on filter opening to mistake proof process; effective 4/3/03 Implementation Identify possible solutions to Root Cause Brainstorm possible solutions Consider both short and long term solutions Consider time required to implement Develop an action plan Determine what information will be monitored and what criteria will be considered acceptable Verification Determine if Corrective Action was successful Review monitoring data and determine if it met success criteria Talk to those involved to see if improvement has been noticed Standardize the Solution Conduct training on process change Remove barriers to make new method efficient Remove all possibilities of reverting to old method