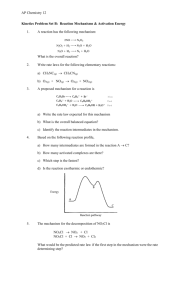
Reaction Mechanism
advertisement

Activation energy Review of Exothermic • Reactants Ep is higher than Products Ep. • Now, we must consider the activation energy (the energy needed so that the reactants bonds will break and reform to make product) Review of Endothermic • Reactants Ep is lower than Products Ep. • Need to add more energy to the system for the forward reaction to take place. • Still need to consider activation energy Activated complex is an unstable chemical species containing partially formed bonds representing the maximum potential energy point in the change -also known as the transition state Activated Complex • Is the short-lived, unstable structure formed during a successful collision between reactant particles. • Old bonds of the reactants are in the process of breaking, and new products are forming • Ea is the minimum energy required for the activation complex to form and for a successful reaction to occur. Fast and slow reactions • The smaller the activation energy, the faster the reaction will occur regardless if exothermic or endothermic. • If there is a large activation energy needed, that means that more energy (and therefore, time) is being used up for the successful collisions to take place. Reaction Mechanism Reaction Mechanisms The overall progress of a chemical reaction can be represented at the molecular level by a series of simple elementary steps or elementary reactions. The sequence of elementary steps that leads to product formation is the reaction mechanism. 2NO (g) + O2 (g) 2NO2 (g) N2O2 is detected during the reaction! Elementary step: NO + NO N 2O 2 + Elementary step: N2O2 + O2 2NO2 Overall reaction: 2NO + O2 2NO2 Reaction Intermediates Intermediates are species that appear in a reaction mechanism but not in the overall balanced equation. An intermediate is always formed in an early elementary step and consumed in a later elementary step. Elementary step: NO + NO N 2O 2 + Elementary step: N2O2 + O2 2NO2 Overall reaction: 2NO + O2 2NO2 Rate Laws and Rate Determining Steps Writing plausible reaction mechanisms: • The sum of the elementary steps must give the overall balanced equation for the reaction. • The rate-determining step should predict the same rate law that is determined experimentally. The rate-determining step is the slowest step in the sequence of steps leading to product formation. Molecularity • The number of molecules that participate as reactants in an elementary step • Unimolecular: a single molecule is involved. – Ex: CH3NC (can be rearranged) – Radioactive decay – Its rate law is 1st order with respect to that reactant • Bimolecular: Involves the collision of two molecules (that form a transition state that can not be isolated) – Ex: NO + O3 NO2 + O2 – It’s rate law is 1st order with respect to each reactant and therefore is 2nd order overall. – Rate =k[NO][O3] Termolecular: simultaneous collision of three molecules. Far less probable. • Some possible mechanisms for the reaction; 2A + B C + D Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular reaction A+B products rate = k [A][B] Bimolecular reaction A+A products rate = k [A]2 A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed. Ea k uncatalyzed catalyzed ratecatalyzed > rateuncatalyzed Energy Diagrams Exothermic Endothermic (a) Activation energy (Ea) for the forward reaction 50 kJ/mol 300 kJ/mol (b) Activation energy (Ea) for the reverse reaction 150 kJ/mol 100 kJ/mol (c) Delta H -100 kJ/mol +200 kJ/mol The experimental rate law for the reaction between NO2 and CO to produce NO and CO2 is rate = k[NO2]2. The reaction is believed to occur via two steps: Step 1: NO2 + NO2 NO + NO3 Step 2: NO3 + CO NO2 + CO2 What is the equation for the overall reaction? NO2+ CO NO + CO2 What is the intermediate? Catalyst? NO3 NO2 What can you say about the relative rates of steps 1 and 2? rate = k[NO2]2 is the rate law for step 1 so step 1 must be slower than step 2 Write the rate law for this reaction. Rate = k [HBr] [O2] List all intermediates in this reaction. HOOBr, HOBr List all catalysts in this reaction. None Reaction mechanisms are only educated guesses at the behaviour of molecules, but there are three rules that must be followed in proposing a mechanisms: 1. each step must be elementary 2. The slowest step must be consistent with the rate equation 3. The elementary steps must add up to the overall equation Temperature Dependence of the Rate Constant k = A e -Ea/RT (Arrhenius equation) Ea is the activation energy (J/mol) R is the gas constant (8.314 J/K•mol) T is the absolute temperature A is the frequency factor -Ea 1 ln k = + lnA R T Note: Changes to both the activation energy and temperature have exponential effects on the value k, hence rate of reaction • The Arrhenius equation show the effect of temperature on the rate constant, k • It indicates that k depends exponentially on temperature Arrhenius equation: k = A e-Ea/RT Ea – activation energy R – gas constant, 8.3145 J mol-1K-1 T - Kelvin temperature A – Arrhenius constant (depends on collision rate and shape of molecule) k=A -Ea/RT e • As T increases, the negative exponent becomes smaller, so that value of k becomes larger, which means that the rate increases. Higher T Larger k Increased rate ln k and 1/T is linear • With R known, we can find Ea graphically from a series of k values at different temperatures. • ln k2 = - Ea ( 1/T2 – 1/T1) k1 R • Ea = -R (ln k2 / k1) ( 1/T2 – 1/T1) -1 Problem • The decomposition of hydrogen iodide has rate constants of 9.51 x10-9 L/mol.s at 500.0 K and 1.10 x 10-5 L/mol.s at 600.0 K. Find Ea. Ea = - (8.314)( ln 1.10 x10-5/ 9.51 x109)(1/600.00 – 1/500.0) = 1.76 x 105 J/mol • If rearrange the equation and convert it to: lnk = - Ea . 1 + lnA R T • A graph of ln k against 1/T will be linear with a slope/gradient of –Ea/R and an intercept on the y-axis of lnA y lnk = - Ea . 1 + lnA R T = m . x +b Plot lnk vs. 1/T = straight line This is known as an Arrhenius plot