Kinetics Problem Set B: Reaction Mechanisms
advertisement

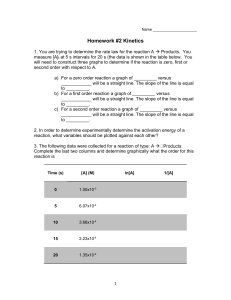
AP Chemistry 12 Kinetics Problem Set B: Reaction Mechanisms & Activation Energy 1. A reaction has the following mechanism: What is the overall reaction? 2. Write rate laws for the following elementary reactions: a) CH3NC(g) CH3CN(g) b) O3(g) + NO(g) O2(g) + NO2(g) 3. A proposed mechanism for a reaction is a) Write the rate law expected for this mechanism b) What is the overall balanced equation? c) Identify the reaction intermediates in the mechanism. 4. Based on the following reaction profile, a) How many intermediates are formed in the reaction A C? b) How many activated complexes are there? c) Which step is the fastest? d) Is the reaction exothermic or endothermic? Energy 5. The mechanism for the decomposition of NO2Cl is NO2Cl NO2 + Cl NO2Cl + Cl NO2 + Cl2 What would be the predicted rate law if the first step in the mechanism were the rate determining step? AP Chemistry 12 6. Show that the following two mechanisms give the same net overall reaction. The following questions are AP specific (i.e. not required for Chemistry 12) 7. The following data were collected for a reaction: Determine the activation energy for the reaction in kJ/mol both graphically and by calculation. For the calculation of Ea, use the first and last sets of data. 8. Rate constants were measured at various temperatures for the reaction HI(g) + CH3I(g) CH4(g) + I2(g). The following data were obtained: Determine the activation energy in kJ.mol both graphically and by calculation. For the calculation of Ea, use the first and last sets of data. Answers: 1. 2NO + 2H2 N2 + 2H2O 2. a) rate = k[CH3NC] 3. a) rate = k[C4H9Br] b) C4H9Br + 2H2O C4H9OH + Br- + H3O+ c) C4H9+ and C4H9OH+ 4. a) 1 5. rate = k[NO2Cl] 6. It works! 7. 79 kJ/mol 8. 139 kJ/mol b) 2 b) rate = k[O3][NO] c) peak between A and B d) exothermic