Arrhenius Equation
advertisement

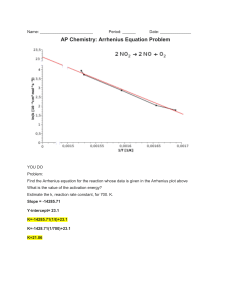
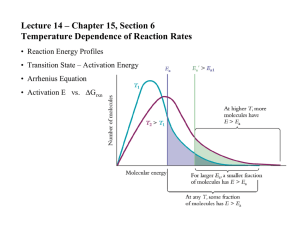
Arrhenius Equation - The Arrhenius equation quantitatively describes the relationship between the rate constant k, temperature and the activation energy. The rate constant value increases with increase in temperature and nothing else varies it! k = A e(-Ea/RT) where k = rate constant (from the rate expression) A = a constant for a given reaction, sometimes called the 'frequency' or 'pre-exponential' factor and it seems to be linked to stereochemical factors i.e. the spatial aspects of reactant particle collision. e = 2.718 or the button on your calculator Ea = activation energy in Jmol-1 R = ideal gas constant = 8.314 Jmol-1K-1 T = temperature in K (Kelvin = oC + 273) - Rewriting the Arrhenius equation in logarithmic form gives: ln(k) = ln(A) - Ea (RT) or log(k) = log(A) – Ea (2.303 RT) - Rearranging this gives you log (k) = (-Ea) __1__ + log (A) (2.303R) T Y= m x +b ** Therefore, plotting log k versus 1/T will give a straight line. - From accurate rate data at different temperatures (e.g. 5 or 10o intervals and a minimum of four results) you can calculate the values of k OR more simply, for a fixed 'recipe', a set of 'rate' results. You then plot the value of ln(k or relative rate) versus the reciprocal temperature in Kelvin. - From this graph, the slope of the line is equal to –Ea/R. To calculate the activation energy required, Ea = -R x slope. - If you plot log k instead of ln k, the slope will be equal to –Ea/(2.303 R) and Ea = -2.303 R x slope. - The Arrenhius Equation can also be manipulated so that it can be used to solve for two different temperatures and two different rate constants. log k2 k1 = Ea 2.303 R (1 - 1) (T1 T2)