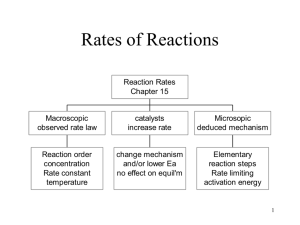
Lecture 18-Kinetics 2

Kinetics pt 2: Temperature Dependence of
Rate Constants
Lecture 18 (Ch 18)
HW: Ch 18: 1, 3, 15, 41
Temperature and Rate
The rates of most chemical reactions increase with temperature.
How is this temperature dependence reflected in the rate expression?
Rates increase with temperature because rate constants increase with temperature.
An example is the 1 st order reaction:
CH
3
NC ---> CH
3
CN 𝒓𝒂𝒕𝒆 = 𝒌[𝑪𝑯
𝟑
𝑵𝑪]
Variation in k with temperature.
Collision Model
The collision model makes sense of this. This model is based on kinetic theory
We’ve seen that the thermal energy of a molecule is converted to kinetic energy in order to facilitate motion, and we’ve seen that the velocity of a molecule increases with T.
The central idea of the collision model is that molecules must collide to react. The more collisions per second, the faster the reaction goes .
This model also rationalizes the concentration dependence. The more molecules present, the more collisions you have .
𝑉 𝑉
Activation Energy
Of course, there is more to a chemical reaction than just collisions of molecules.
Molecules must have some minimum energy in order to overcome the energy barrier of reaction
Upon colliding, the kinetic energy of molecules is used to stretch, bend, and break bonds in order to cause a reaction.
But if the molecules don’t have enough kinetic energy, they simply bounce off one another.
𝑉 𝑉 𝑉
E too low
This minimum energy that molecules must have is called the activation
energy,(E a
)
𝑉
Activation Energy Needed to Reach Transition State
• Lets go back to the reaction
CH
3
NC ---> CH
3
CN
• The molecule passes through a transition state , a high-energy
intermediate in which the CN bond gets rotated 90 o .
Transition state
• This is an unstable configuration and requires an activation energy to be reached.
ΔH nitrogen carbon
• Once this transition state is reached, the process is energetically downhill. The transition state is always the highest energy point in the reaction pathway. As drawn, this reaction is exothermic because the energy of the products is less than the energy of the reactants .
Arrhenius Equation
The fraction of molecules that have an energy equal to or greater than the activation energy is given by the expression: 𝒇 = 𝒆
−
𝑬 𝒂
𝑹𝑻
Arrhenius noted that reaction-rate data depended on three aspects
(1) the fraction of molecules possessing an energy E a or greater
(2) the number of collisions per second
(3) the fraction of molecules oriented in the right way for a reaction to occur
These factors are incorporated into the Arrhenius Equation 𝐤 = 𝐀𝒆
−
𝑬 𝒂
𝑹𝑻
This equation relates k to temperature. A is the frequency factor , and is related to aspects (2) and (3) listed above.
Rearranging the Arrhenius Equation
Taking the natural log of both sides, the Arrhenius equation becomes:
𝑬 𝒂 𝐥𝐧 𝒌 = −
𝑹𝑻
+ 𝐥𝐧 𝑨
• As you see, we once again have an equation in y=mx+b form. y = ln k 𝑚 𝑠𝑙𝑜𝑝𝑒 = −
𝐸 𝑎
𝑅
1 𝑥 =
𝑇
Plotting ln k vs 1/T yields a linear plot with slope –E a
/R and a yintercept of ln A
Showing Changes in k Graphically
𝐥𝐧 𝒌 = −
𝑬 𝒂
𝑹𝑻
+ 𝐥𝐧 𝑨
From the data below, determine the activation energy of the 1 st order reaction
Temperature, o C K (s -1 )
189.7
2.52 x 10 -5
198.9
230.3
251.2
5.25 x 10 -5
6.30 x 10 -4
3.16 x 10 -3
1/T ( o K -1 ) ln K
0.002161
0.002119
0.001987
-10.5887
-9.8547
-7.36979
0.001908
-5.75718
Plot ln k vs 1/T to get the slope. We know R, so we can calculate E a
. T must be in Kelvin.
0
0,00185 0,0019 0,00195 0,002 0,00205 0,0021 0,00215 0,0022
-2
-4
-6
-8 y = -19038x + 30,517
-10
-12
1/T 𝑠𝑙𝑜𝑝𝑒 = −19038 = −
𝐸 𝑎
𝑅
= −
(8.314
𝐸 𝑎
𝐽 𝑚𝑜𝑙 𝐾
)
𝑬 𝒂
𝑱
= 𝟏. 𝟓𝟖 𝒙 𝟏𝟎 𝟓 𝒎𝒐𝒍
Calculating Changes in k Mathematically
The Arrhenius equation can be rearranged once again to compare k values at different temperatures ln 𝑘
2 𝑘
1
=
𝐸 𝑎
𝑅
𝑇
2
− 𝑇
1
𝑇
1
𝑇
2
Please note the following: 𝑒 ln(𝑥) = 𝑥
Example
The reaction above has an activation energy of 43.5 kJ/mol. The reaction occurs at 298 K with a rate constant of 110 s -1 . What will the rate constant be if we increase the temperature to 308 K?
ln 𝑘
2 𝑘
1
=
𝐸 𝑎
𝑅
𝑇
2
− 𝑇
1
𝑇
1
𝑇
2 ln 𝑘
2
110
=
43500 𝐽 𝑚𝑜𝑙
−1
8.314 𝐽 𝑚𝑜𝑙 −1 𝐾 −1
308 − 298
91784 𝑘
2 ln
110
= .57
𝑒 𝑙𝑛 𝑘
2
110 = 𝑒 .57
𝑘
2
110
= 1.77
𝑘
2
= 194.7 𝑠 −1
Reaction Mechanisms
In a previous lecture, I alluded to the following reaction:
𝑵𝑶
𝟐 𝒈 + 𝑪𝑶 𝒈 → 𝑵𝑶(𝒈) + 𝑪𝑶
𝟐
(𝒈)
In looking at this equation, and applying your understanding of collision theory, you would assume that this reaction proceeds when
NO
2 and CO collide
The activation energy is exceeded
The atoms rearrange to form NO and CO
2
• Thus, we would expect the rate of the reaction to depend on both [NO
2
] and [CO]
Reaction Mechanisms
𝑵𝑶
𝟐 𝒈 + 𝑪𝑶 𝒈 → 𝑵𝑶(𝒈) + 𝑪𝑶
𝟐
(𝒈)
However, according to experimental data, the reaction is 2 nd order with respect to NO
2 and zero order with respect to CO 𝑟𝑎𝑡𝑒 = 𝑘[𝑁𝑂
2
] 2 [𝐶𝑂] 0 = 𝑘[𝑁𝑂
2
] 2
• This means that the rate of the reaction does not depend on [CO] at all.
How can this be? What does this tell us about the reaction mechanism ?
• This particular reaction must proceed through multiple steps, and the rate-determining step must only depend on NO
2
Reaction Mechanisms
𝑵𝑶
𝟐 𝒈 + 𝑪𝑶 𝒈 → 𝑵𝑶(𝒈) + 𝑪𝑶
𝟐
(𝒈)
• For this particular reaction, there are two steps:
1 𝑁𝑂
2 𝑔 + 𝑁𝑂
2 𝑔 → 𝑁𝑂
3 𝑔 + 𝑁𝑂 𝑔 (𝑠𝑙𝑜𝑤)
2 𝑁𝑂
3 𝑔 + 𝐶𝑂 𝑔 → 𝑁𝑂
2 𝑔 + 𝐶𝑂
2 𝑔
3 𝑁𝑂
2 𝑔 + 𝐶𝑂 𝑔 → 𝑁𝑂 𝑔 + 𝐶𝑂
2 𝑔
(𝑣𝑒𝑟𝑦 𝑓𝑎𝑠𝑡)
• This step-by-step description of the molecular pathway is the reaction mechanism . From reaction (1), we can physically understand why the reaction is 2 nd order with respect to [NO
2
].
• The 1 st step is substantially slower than the 2 nd . Therefore, step (1) acts as the “reaction bottleneck”, and the overall reaction speed can only be as fast as its slowest step .
• Thus, step (1) is the rate determining step .
Each step in a reaction mechanism is called an elementary reaction .
An elementary reaction is any reaction that proceeds in a single step.
Mechanisms consist of sums of elementary reactions .
Going back to rate laws, each elementary reaction must have a corresponding rate constant
1 𝑁𝑂
2
2 𝑁𝑂
3 intermediate (transition step) 𝑔 + 𝑁𝑂
2 k 𝑔 −−→ 𝑁𝑂
3 k 𝑔 + 𝐶𝑂 𝑔 −−→ 𝑁𝑂
2 𝑔 + 𝑁𝑂 𝑔 𝑔 + 𝐶𝑂
2 𝑔
(𝑠𝑙𝑜𝑤)
(𝑣𝑒𝑟𝑦 𝑓𝑎𝑠𝑡)
*** For elementary reactions only, you can assume that the rate law depends directly on the number of species present. Thus, the rate law of
an elementary reaction follows the stoichiometry.
𝑟𝑎𝑡𝑒
1
= 𝑘
1
𝑁𝑂
2
𝑁𝑂
2
= 𝑘
1
[𝑁𝑂
2
] 2 rate of overall reaction
Reaction Mechanisms Involving a Fast Equilibrium Step
Let’s consider reaction mechanisms with a fast equilibrium step.
To date, we have discussed reactions occurring in only one direction.
However, in many cases, the forward and back reactions are both important. For example: k
1
2𝑁𝑂 𝑔 𝑁
2
𝑂
2
(g) k
-1
• When a reaction is in equilibrium, like the one shown above, the forward and back rates are equal, so there is no change in the amount of reactant or product once equilibrium is established.
Reaction Mechanisms Involving a Fast Equilibrium Step
For many reaction mechanisms, a rapid equilibrium step is involved. Lets look at the following reaction:
2𝑁𝑂 𝑔 + 𝑂
2 𝑔 → 2𝑁𝑂
2
(𝑔)
This reaction goes through two elementary steps:
1 2𝑁𝑂 𝑔
2 𝑁
2
𝑂
2 𝑔 + 𝑂
2 k
1
𝑁
2
𝑂
2 𝑔 (𝑣𝑒𝑟𝑦 𝑓𝑎𝑠𝑡) k
-1 𝑔 −−→ 2𝑁𝑂
2 𝑔 (𝑠𝑙𝑜𝑤)
Overall reaction rate would be written as: 𝑟𝑎𝑡𝑒 𝑟𝑥𝑛
= 𝑘
2
𝑁
2
𝑂
2
[𝑂
2
]
[N
2
O
2
] is an intermediate . Intermediates are never included in the overall rate law because they are too short-lived, so their concentrations can’t easily be measured.
Mathematical Substitution For [Intermediate]
1 2𝑁𝑂 𝑔
2 𝑁
2
𝑂
2 𝑔 + 𝑂
2 k
1 k
-1 𝑔 k
2
𝑁
2
𝑂
2
2𝑁𝑂
2 𝑔 (𝑣𝑒𝑟𝑦 𝑓𝑎𝑠𝑡) 𝑔 (𝑠𝑙𝑜𝑤) 𝑟𝑎𝑡𝑒 𝑟𝑥𝑛
= 𝑘
2
𝑁
2
𝑂
2
[𝑂
2
]
• Since we have an equilibrium in step (1), the forward and back rates are equal 𝑘
1
[𝑁𝑂] 2 = 𝑘
−1
[𝑁
2
𝑂
2
]
• Solving for [N
2
O
2
], we get: 𝑘
1 𝑘
−1
[𝑁𝑂] 2 = [𝑁
2
𝑂
2
]
• Substitute this term into our rate law expression: 𝑟𝑎𝑡𝑒 𝑟𝑥𝑛
= 𝑘
2 𝑘
1 𝑘
−1
[𝑁𝑂] 2 [𝑂
2
] may be written in the book as simply K
Examples
Write the rate law for the following reaction given the elementary reaction steps of the mechanism:
2𝑁𝑂
2 𝑔
1 𝑁𝑂
2
2 𝑁𝑂
3 𝑔 + 𝑁𝑂
2 𝑔 k
2 𝑔
2𝑁𝑂 𝑔 + 𝑂
2
(𝑔) k
1
𝑁𝑂 𝑔 + 𝑁𝑂
𝑁𝑂 𝑔 + 𝑂
2 𝑔
3 𝑔 (𝑠𝑙𝑜𝑤)
(𝑓𝑎𝑠𝑡)
• Write the rate law for the following reaction given the elementary reaction steps of the mechanism. Substitute any intermediates .
2𝑁𝑂 𝑔 + 𝐻
2 𝑔 k
1
1 2𝑁𝑂 𝑔
2 𝑁
2
𝑂
2 k
-1 𝑔 + 𝐻
2 𝑔
𝑁
2
𝑂 𝑔 + 𝐻
2
𝑂 𝑔 k
2
𝑁
2
𝑂
2 𝑔 (𝑣𝑒𝑟𝑦 𝑓𝑎𝑠𝑡)
𝑁
2
𝑂 𝑔 + 𝐻
2
𝑂(𝑔) (𝑠𝑙𝑜𝑤)