Laura Yu, Wendy Zeng, Anthony Wong Chemistry 5° Ch. 12 + 14
advertisement
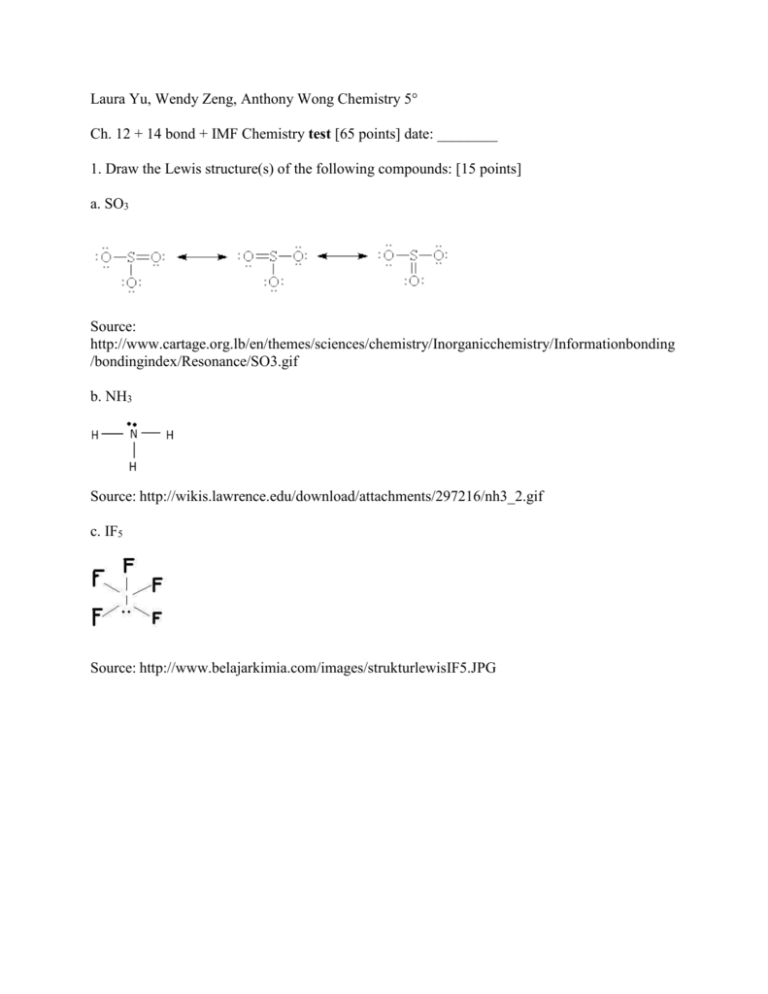
Laura Yu, Wendy Zeng, Anthony Wong Chemistry 5° Ch. 12 + 14 bond + IMF Chemistry test [65 points] date: ________ 1. Draw the Lewis structure(s) of the following compounds: [15 points] a. SO3 Source: http://www.cartage.org.lb/en/themes/sciences/chemistry/Inorganicchemistry/Informationbonding /bondingindex/Resonance/SO3.gif b. NH3 Source: http://wikis.lawrence.edu/download/attachments/297216/nh3_2.gif c. IF5 Source: http://www.belajarkimia.com/images/strukturlewisIF5.JPG 2. fill-in the below table. [10 points] # bonding pair of electrons 4 2 # nonbonding Shape of pair of electrons molecule 1 3 see-saw Linear Bond angle(s) 90°,120°,180° 180° 3. Sketch the shape of the molecules. What is the name of the shape of the molecules ? What are the bond angles in the molecules ? [hint: see # 1, above; 15 points] a. SO3 trigonal planar; 120° b. NH3 trigonal pyramidal;109.5° c. IF5 square pyramidal;90° 4. At room temperature, water is a liquid while carbon dioxide is a gas. Rationalize this observation using concepts involving intermolecular forces. [10 points] H2O is a polar compound with H-bond IMF and CO2 is non-polar with London Dispersion IMF. H2O has greater IMF, than CO2, therefore it has a lower vapor pressure. So, at room temperature it remains a liquid and CO2 is a gas. 5. Which of the following chemicals: NH3, CH4, CI4 has the highest vapor pressure ? lowest vapor pressure ? Justify your answer. [15 points] Since, NH3 has H-Bond IMF it is greater than both CH4 and CI4, which have London IMF’s, but CH4 has an London Dispersion making it have greater forces than CI4, which has no H-bond. The higher the IMF, the lower the vapor pressure, as it is harder to separate, therefore lower evaporation, thus the low vapor pressure. So, CI4 has the highest vapor pressure, because it has the lowest IMF and NH3 has the greatest vapor pressure, because it has the highest IMF. CH4 is methane, which means it is a gas, meaning it is easier to evaporate, so vapor pressure is higher. IMF: NH3 > CH4 > CI4 VO: CI4 > CH4 > NH3








