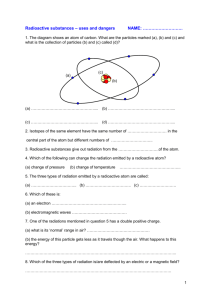
Radiometric Dating
advertisement

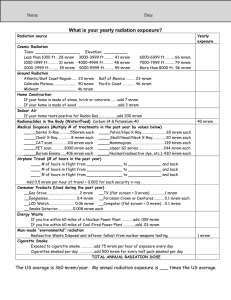
Radiometric Dating “clocks in rocks” Absolute Dating Gives a numerical age Works best with igneous rocks & fossils Uses isotopes Isotopes- different number of neutrons Carbon 14 (14C) – 2 “extra” neutrons – radioactive Common dating isotopes Parent Daughter Half life Potassium 40 Argon 40 1.3 by Rubidium 87 Strontium 87 48.8 by Uranium 235 Lead 207 .7 by Carbon 14 Nitrogen 14 6,000 years Sodium 22 Aluminum 27 15 hours radioactivity Nuclei break apart Emit particles or waves (radiation) Radiation Radioactive Atom Ionizing Radiation Alpha Particle Neutron Particle Beta Particle Gamma Ray (X Ray) Average Annual Dose Cosmic 28 mrem Terrestrial 28 mrem Radon 200 mrem Internal 40 mrem Medical X-Rays 40 mrem Nuclear Medicine 14 mrem Natural Sources Man-Made Sources Other 3 mrem Consumer Products 10 mrem Comparison of Radiation Dose 1 10 100 1,000 10,000 100,000 1,000,000 Lethal Dose Radiation Worker Limit Argonne Control Limit Natural Background Average ANL Radiation Worker General Employee Limit Chest X-Ray Doses shown in bar graph are in units of mrem Half-Life The time required for the amount of radioactive material to decrease by one-half 1200 1000 800 Activity 600 400 200 0 New 1 HalfLife 2 HalfLives 3 HalfLives 4 HalfLives Half-lives Parent Daughter Half life Potassium 40 Argon 40 1.3 by Rubidium 87 Strontium 87 48.8 by Uranium 235 Lead 207 .7 by Carbon 14 Nitrogen 14 6,000 years Sodium 22 Aluminum 27 15 hours Half life problem If you begin with 80 grams of 14C after a time, 20 grams are left. How old is the sample? 14 Carbon Only accurate for ages less than 100,000 years Parent gets too small to accurately measure Mass spectrometer measures D/P ratio Preparing a rock for mass spectrometer Using daughter/parent ratio 1) D/P ratio 2) figure out number of half-lives – Use graph 3) multiply number of half-lives by the time of one half-live Example: 240 g 14N ;8g 14C Another half-life problem 1,000 grams of radioactive element is in a rock when it is formed. The element’s half-life is 2 million years. After a time, 125 grams of the original element remain. How old is the rock?