Chapter 6
advertisement

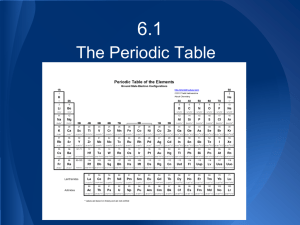
Chapter 6 The Periodic Table S Valence Electrons S These are electrons in the outermost shell (or energy level) for a particular atom. S They are the ONLY electrons that are involved in chemical bonding and chemistry. S Valence electrons can be S Lost S Gained S Shared S Every atom always wants to have 8 of them. 8 is called an “octet.” That’s the “octet rule.” Lewis Structures http://www.ausetute.com.au/lewisstr.html S A Lewis structure can have a maximum of 8 dots. S You put one dot on each side of the symbol (top, bottom, left and right), until each side has a dot. S Then you can start pairing them up, until every side has 2 dots. S When every side has two dots, you can’t put any more dots on the structure. If you need to, you did something wrong. Exceptions to the Rules for electron configurations S Copper and Chromium do not follow the rules. By not following the rules, copper and chromium form a more stable configuration than would be predicted by the rules. S A half full set of p or d orbitals is a stable configuration. A full set of p or d orbitals is even MORE stable. S This is why the Nobel gases are completely unreactive. They have a full set of valence electrons and don’t want or need yours. S It also explains WHY Zinc only has a charge of +2, but we’ll get to that in just a second. Shorthand electron configurations S Use the NOBEL GAS which precedes the element. S Remove the electron configuration for the Nobel gas from your configuration and replace it with [NG] where NG = the symbol for the specific Noble gas. S Let’s do a few examples… The Periodic Law S When you arrange the elements in order of increasing atomic number, there is a periodic repetition of chemical and physical properties. S Write that into your notes in YOUR OWN WORDS. Metals and Nonmetals and Metalloids…oh my! S Metals S Nonmetals S Metalloids (B, Si, Ge, As, Sb, Te, Po) Alkali Metals S Sodium and potassium and all the rest of the elements in that group are alkali metals. S The alkali metals all have one valence electron. That similarity is what makes them behave the same chemically. S They are very reactive. Reactivity is highest on the outer edges of the table and elements get less reactive the closer they are to the center of the table. Lithium is the least reactive alkali metal and reactivity increases as you go down the group. Alkaline Earth Metals & Halogens S Group IIA or Group 2 are called “the alkaline earth metals.” They have 2 valence electrons. S Group VIIA or Group 17 are called “the halogens.” The halogens all have 7 valence electrons, and like the alkali metals, they are very reactive (fluorine is most reactive and reactivity decreases as you go down the group). S Iron is one of the least reactive elements known. It can take literally years for it to react. Noble Gases S The noble gases are very stable. They are unreactive because they are so stable. S The noble gases all have 8 valence electrons. Helium is an exception in that it only has 2. S The noble gases are gases at STP S STP = Standard Temperature and Pressure Representative Element & Transition Metals. S The representative elements are the Group A elements. S The representative elements always behave the same. And any one member of the group is “representative” of all the other members in its group. S The representative elements are all the elements in the s and p blocks. S The transition metals are the Group B elements. S They behave differently at different times. They do not always act the same. S The f block is the Lanthanides (top row) and Actinides (bottom row). S These are also called the “inner transition metals.” Representative Element & Transition Metals. SLet’s Label the Periodic Table. Periodic Trends S Atomic Radius S Decreases from left to right in period (because of increased shielding) S Increases as you go down a group (because you are going to higher energy levels, which are further away from the nucleus). S Ionic Radius S Same as atomic radius S Big change in size between groups 14 and 15, where you shift from positive ions to negative ions. What is an Angstom (Å) http://intro.chem.okstate.edu/1314F00/Lecture/Chapter7/ATRADIID.DIR_PICT0003.gif S Even the largest atoms are very small. The diameter of a uranium atom is only about 0.345 nanometers. 2,898,550,725 uranium atoms could fit on a METER stick!! S A special unit is sometimes used to describe lengths at the atomic level, such as atomic radius or atomic diameter. Note the trend as you go across a row and down a column. S That is the Angstrom. We use a Å to represent Angstroms (if you want to type that it’s shift-alt-A on a Mac and control-shift-2, shift-A on a bogus, inferior, Windows or Vista based machine). Angstoms (Å) http://upload.wikimedia.org/wikipedia/commons/1/11/Hydrogen_Atom.jpg S Even the largest atoms are very small. The diameter of a uranium atom is only about 0.345 nanometers. S 0.345 nm = 3.45Å S 1nm = 10Å S 1Å = 1 x 10-10 meters S A hydrogen atom is the smallest atom. H has a diameter of only 0.74Å. About 13.5 TRILLION hydrogen atoms could fit onto the edge of a meter stick. 2 more Periodic Trends S Ionization Energy S Increases from left to right in period (OPPOSITE from atomic radius and ionic radius) S Decreases as you go down a group (because it gets easier to remove the electron the further away it is from the nucleus. S Electronegativity (how strong an atom attracts electrons to itself). S Same as ionization energy in terms of trends. S DRAW ARROWS on your periodic table to represent trends! Allotropes http://chemistry.about.com/od/periodictableelements/ig/Element-Photo-Gallery.--98 S Allotropes = different forms of the same element. Different structures with different properties. S Diamond and graphite are both forms of carbon. S Oxygen (O2) and ozone (O3) are both allotropes of oxygen. l Diamond and graphite are both allotropes of carbon. Remember Distillation? /activity/distil.htm S Distillation can be used to separate a mixture of liquids based on boiling points. S This is a distillation setup. Organic Flavor S Carbon can bond and form long chains S Like in soap molecules S Carbon can form rings S Like in sugar molecule S Carbon can form huge networks of carbon atoms S Like in diamonds S Carbon is always bonded to 4 things. Carbons unique size (atomic radius) and electronegativity (we’ll get to that in a minute) means that it can form very strong COVALENT bonds between itself and H and O and N and other atoms (that means electrons are SHARED). The End What’s Next…. For Advanced…we still have Chapter 25 to do for Unit 2 For Chemistry…see you in Unit 3 (Chapter 7) S