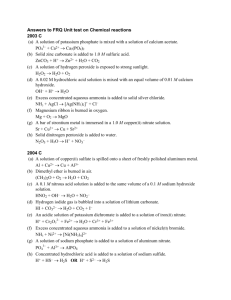
Reactions
advertisement

Reactions 8 Every AP test has a few reaction questions. Given some reactants, you must write a balanced, net ionic equation. We will start with unbalanced equations. Net Reactions Most materials in AP reactions are dissolved. You don't need to figure this out; the problem says so. In such cases all you write is the ion which reacts. Thus when sodium chloride solution reacts with silver nitrate solution, you write: Ag+ + Cl– AgCl The sodium and the nitrate ions are spectators and are not included. Sometimes materials are added as solids. Generally this is stated explicitly, as in: "solid zinc nitrate," or "a suspension of copper(II) hydroxide." Sometimes, however, you know it's a solid because it isn't a solution, as in "excess silver acetate is added to potassium chloride solution." In such cases you write out the whole compound, and not just the part that reacts. AgC2H3O2 + Cl– AgCl + C2H3O 2– Note that the potassium is a spectator and is omitted. However, the acetate ion has to be included on the right because it was part of the solid on the left. Although we are starting with unbalanced equations, any element which is on one side of an equation must also appear on the other. The following equation is incorrect because it has no oxygen on the right: CrO42– + Fe2+ Cr3+ + Fe3+ But you can't just add O2. That would mean than oxygen was going from an oxidation state of -2 (in chromate) to an oxidation state of 0 in oxygen gas. Instead you have to add water to the right. This, then, means you have to add H+ to the left, giving you: CrO42– + Fe2+ + H+ Cr3+ + Fe3+ + H2O To make things easier, the reactions have been divided into categories. These categories are arbitrary, and could have been done differently. But I doubt that it would be VERY different. Writing reactions when you know the category is fairly easy. You will start by learning reactions within their category. Then, after you have had time to practice, we will start mixing the categories. Precipitation Reactions These are reactions in which a precipitate (an insoluble material) forms. To do them you must know the solubility rules. (A handout which you have received). What is important here is not what is soluble but what is insoluble, since it is the insoluble materials that form precipitates. You need to know the insoluble materials in Rule 3 (silver and lead halides), Rule 4 (calcium, barium and lead sulfates), and rule 5 (most hydroxides, sulfides, sulfites, carbonates, phosphates and chromates). So if barium nitrate solution is mixed with potassium sulfate solution, Rule 4 says that barium sulfate is insoluble and: -1- Ba2+ + SO42– BaSO4 People worry about what to do with the slightly soluble (Rule 5) calcium and barium hydroxides. Don't. When they are reactants, the question will always make clear whether they should be treated as soluble or insoluble. Thus, mixing solutions of calcium hydroxide and sodium phosphate would give insoluble (Rule 5) calcium phosphate. Since it says "...solutions of calcium hydroxide..." Ca2+ + PO43– Ca3(PO4)2 When calcium or barium hydroxides are products, they are considered insoluble. Some Examples Solutions of potassium carbonate and zinc nitrate are mixed. Zn2+ + CO32– ZnCO3 A solution of copper(II) sulfate is added to a solution of barium hydroxide. Cu2+ + SO42– + Ba2+ + OH– Cu(OH)2 + BaSO4 Hydrogen sulfide gas is bubbled through a solution of lead(II) nitrate. H2S + Pb2+ PbS + H+ Acid/Base Reactions You need to know the strong acids -- HCl, HBr, HI, HNO3, H2SO4. These are considered to be completely dissociated. All other acids are weak and are written undissociated. You also need to know that an acid and a base react to form water HF + OH– H2O + F– and that the anion from a weak acid is protonated by water (or H+). NO–2 + H2O HNO2 + OH– Some reactions specify certain numbers of moles of acid and base. This usually leads to the partial protonation of a polyprotic acid. Thus if sodium phosphate is mixed with an equal number of moles of hydrochloric acid solution: H+ + Na3PO4 Na+ + HPO42– You should know that a carbonate (or bicarbonate) reacts with acid to form carbon dioxide and water. CaCO3 + H+ Ca2+ + CO2 + H2O This is because protonation of the carbonate ion forms H2CO3, which comes apart to form CO2 and H2O. Sulfides react with acid to form hydrogen sulfide: Fe2S3 + HC2H3O2 Fe+3 + C2H3O–2 + H2S Finally, the oxides and hydroxides of certain metals, called amphoteric metals, can react as if they are either acids or bases. The only amphoteric metals you need to remember are zinc and aluminum. Thus zinc hydroxide dissolves in sodium hydroxide solution: Zn(OH)2 + OH– Zn(OH)42– Every once in a while the AP exam asks about a gas phase Lewis acid/base reaction: BF3 + NH3 F3B–NH3 Every question I've seen has used a boron trihalide or trihydride as the Lewis acid. -2- Some More Examples A solution of sodium hydroxide is added to a solution of ammonium chloride. OH– + NH4+ NH3 + H2O Dilute hydrochloric acid is added to a potassium carbonate solution. H+ + CO32– H2O + CO2 Sodium hydroxide solution is added to a precipitate of aluminum hydroxide. Al(OH)3 + OH– Al(OH)63– Dilute solutions of acetic acid and ammonia are mixed. HC2H3O2 + NH3 NH4+ + C2H3O–2 Redox Reactions In a redox reaction one species is oxidized (its oxidation state becomes more positive) and another species is reduced (its oxidation state becomes less positive). To do these problems, you must know the common oxidizing and reducing agents mentioned in Table 3 -- Some Useful Reactions. You should realize that oxidizing agents are themselves reduced, while reducing agents are oxidized. It sounds funny but it makes sense. It helps if you are familiar with the common oxidizing and reducing agents. For example, hydrogen peroxide is usually an oxidizing agent (and is therefore reduced): H2O2 + Fe2+ H2O + Fe2+ However, it can occasionally be a reducing agent (and be oxidized): H2O2 + Cr2O72– O2 + Cr3+ + H+ Look at the way the oxidation state of oxygen changes in these reactions. Permanganate, chlorate, bromate, iodate, chlorine, bromine or oxygen are always oxidizing agents. Other oxidizing agents are less certain because they can do other things besides oxidize. You must further remember that permanganate forms Mn2+ in acidic solutions and MnO2 in basic solutions. MnO–4 + I– + H+ Mn2+ + I2 + H2O MnO–4 + SO32– + OH– MnO2 + H2O + SO42– If the acidity or alkalinity is not specified, assume an acid solution. Some More Examples A solution of tin(II) sulfate is mixed with acidified potassium dichromate. Sn2+ + Cr2O72– + H+ Sn4+ + Cr3+ + H2O Hydrogen peroxide solution is added to a solution of iron(II) sulfate H2O2 + Fe2+ H2O + Fe3+ In grading: "RWO" will mean reduction without oxidation; "OWR" will mean oxidation without reduction. -3- Metal Displacement Reactions These are a particular type of redox reaction in which a metal, in its elemental state is oxidized. Usually this combines with another metal being reduced. (Thus one metal displaces another.) If a piece of copper is put into a silver nitrate solution, the copper replaces the silver: Cu + Ag+ Cu2+ + Ag In real life you would have to know which metal is more active in order to determine whether the replacement reaction goes. But here, all the reactions go. So you need not worry about the activity series. Also I've included the reaction of metals with acid or water, since the reaction is the same except now it's hydrogen which is reduced. So the reaction of aluminum with hydrochloric acid is: Al + H+ Al3+ + H2 And the reaction of calcium with water is: Ca + H2O Ca(OH)2 + H2 Metal displacement reactions are easy to recognize because they involve a piece of metal. Dissolving metals in nitric acid or in hot sulfuric acid is more complicated, since these acids also act as oxidizing agents. In each case the nitrogen or sulfur is reduced to the oxide of a lower oxidation state. This question usually involves copper, lead, or silver. These metals are less reactive than H+ and, therefore, too inert to dissolve in ordinary acids (e.g. HCl). They need the additional oxidizing power of the (acidified) nitrate or the (hot, acidified) sulfate. H+ + NO–3 + Ag Ag+ + H2O + NO (or NO2) H+ + SO42– + Cu Cu2+ + H2O + SO2 Some More Examples A piece of aluminum metal is added to silver acetate solution. Al + Ag+ Al3+ + Ag Combustion These are easy because the questions tells you that something is being burned. You take the material being burned, add oxygen gas, and convert the carbon to carbon dioxide and the hydrogen to water. C2H5OH + O2 CO2 + H2O If they are present, chlorine is converted to HCl and most other elements are converted to their oxides. Some More Examples Carbon disulfide vapor is burned in oxygen. CS2 + O2 CO2 + SO2 Gaseous silane (SiH4) is burned in oxygen. SiH4 + O2 SiO2 + H2O -4- Reaction with Water We are principally concerned about oxides reacting with water. Metal oxides react to form hydroxides. In fact, forming basic hydroxides is considered to be a property of metal ions and is shown on some periodic tables by a blue color. So adding water to potassium oxide would give: K2O + H2O K+ + OH– Non-metal oxides, however, react with water to form acids. They are often called "acid anhydrides" and are shown by a red color on some periodic tables. Several oxides which form acidic solutions are shown on the "Some Useful Reactions" sheet, but one example is sulfur dioxide: SO2 + H2O H2SO3 If you are paying attention, you will have no trouble recognizing a non-metal oxide and knowing that it forms an acid. But which acid does it form? Sulfur dioxide was easy because you added one molecule of sulfur dioxide to one molecule water to form one molecule of sulfurous acid. But suppose the question asked about N2O5? You know two nitrogen oxy-acids, HNO3 and HNO2. Which one does it form? This type of question is answered by determining the oxidation state of the non-metal and then finding which acid has the non-metal in the same oxidation state. In N2O5 nitrogen is in the +5 oxidation state. This matches with the N(+5) in HNO3. Using similar logic: P4O10 + 6 H2O 4 H3PO4 SO3 + H2O H2SO4 Although oxides are the most important cases, nitrides react similarly: Mg3N2 + H2O Mg(OH)2 + NH3 This actually makes sense if you realize that the nitride anion reacts like the anions of other weak acids. It doesn't matter whether you deal with oxide, nitride, hydride, fluoride, acetate or any other such anion. The anion removes protons from water, leaving hydroxide ions. Thus LiH + H2O Li+ + H2 + OH– KF + H2O K+ + HF + OH– And calcium carbide, which unexpectedly contains the C22– anion, reacts as shown below. CaC2 + H2O Ca2+ + C2H2 + OH– Remember this! Some More Examples Carbon dioxide is bubbled through a sodium hydroxide solution. CO2 + OH– CO32– + H2O Sodium phosphide is dissolved in water. Na3P + H2O Na+ + H3P + OH– -5- Complexation A complex is a compound which forms between a metal and an electron donor called a "ligand." In AP reactions, the ligand is usually either ammonia or cyanide. Thus, if solutions of sodium cyanide and silver nitrate are mixed: Ag+ + CN– Ag(CN)–2 The negative charge on the complex ion is the sum of one plus (for the Ag+) and two minuses (for the CN–). If a concentrated ammonia solution is added to a suspension of zinc hydroxide: Zn(OH)2 + NH3 Zn(NH3)42+ Notice that the number of ligands, called the coordination number, varies. If you don't know otherwise you should assume the coordination number is twice the metal's charge. One special complexation reaction you should know is the reaction of iron(III) with thiocyanate (SCN–): Fe3+ + SCN– Fe(SCN)2+ There's really more to this reaction than you see, but this is enough. Still another special case is the amphoteric metals, aluminum and zinc, dissolving in base. The products of this reaction can be viewed as hydroxide complexes: Al(OH)3 + OH– Al(OH)63– Complexes in which the ligand can be protonated to form a weak acid (e.g. NH3 and CN–) come apart in acid solution. For example: Cu(NH3)42+ + H+ Cu2+ + NH4+ Some More Examples A suspension of copper(II) hydroxide in water is treated with excess ammonia. Cu(OH)2 + NH3 Cu(NH3)42+ + OH– Electrolysis The good thing about electrolysis reactions is that the questions tells you that electrolysis is occurring. The bad thing is that even then some of the reactions are hard to figure out. The most important thing to remember, though, is that you need both a reduction and an oxidation. Don't forget! If a solution of potassium iodide is electrolyzed, you get: I– + H2O I2 + H2 + OH– Oxidizing iodide is easy. But what is reduced? Potassium is much too difficult to reduce and hydrogen is all that is left. If a dilute solution of sulfuric acid is electrolyzed, you get: H2O H2 + O2 Here there is nothing which can be oxidized other than oxygen. Actually the sulfuric acid isn't a reactant; it just makes the solution conductive. But the sulfate confuses many students. You must realize that sulfate (where S is +6) cannot be oxidized and is usually not reduced. -6- Some Examples A solution of copper(II) sulfate is electrolyzed using platinum electrodes. Cu2+ + H2O Cu + O2 + H+ Again, there is nothing in the solution except for oxygen which could be oxidized. The platinum is there to show you that the electrodes are non-reactive. Decomposition When a compound reacts all by itself (usually with heat) it is decomposing. Some carbonates and hydroxides do this. Thus, if calcium carbonate is heated: CaCO3 CaO + CO2 If magnesium hydroxide is heated: Mg(OH)2 MgO + H2O But what happens when potassium nitrate is heated? Neither nitrogen oxides nor K3N are very stable. So: 2 KNO3 2 KNO2 + O2 The 2002 AP test asked what happened when sodium bicarbonate was heated. This was tricky because sodium carbonate does not decompose easily. Even knowing that, you still had to balance the equation to figure out what happens: NaHCO3 Na2CO3 + H2O + CO2 Most of the decomposition reactions which you will see involve ionic solids. They generally form other ionic solids and NOT the ions themselves. Separate ions (e.g. Ca2+) exist only in solution. Look at the examples above and you will what this means. Some Examples Hydrogen peroxide solution is warmed. H2O2 H2O + O2 Combination If heating a solid in an atmosphere of a specific gas causes it to react, the reaction is probably a combination reaction. Thus heating calcium oxide in sulfur dioxide: CaO + SO2 CaSO3 Similarly if magnesium metal is heated in nitrogen gas: Mg + N2 Mg3N2 If titanium metal is heated in an atmosphere of oxygen: Ti + O2 TiO2 Some Examples Excess chlorine gas is passed over hot iron filings. Fe + Cl2 FeCl3 Also acceptable would be FeCl2. -7- Some Other Important Reactions Know the reactions in the last section of the "Some Useful Reactions" handout. They don't fit into a category well, but they are important and have each been asked several times. Some Examples Chlorine gas is bubbled into a cold, dilute solution of potassium hydroxide. Cl2 + OH– OCl– + Cl– + H2O Copper(II) oxide is heated in an atmosphere of hydrogen. CuO + H2 Cu + H2O Aluminum metal is placed in a solution of potassium hydroxide. Al + OH– Al(OH)6-3 + H2 Aluminum really does react with base the same way it reacts with acid. This was once a common laboratory method for generating hydrogen. Catalysis If your reaction employs a catalyst, don't let it worry you. You can leave it out of the reaction, because catalysts are not consumed. Thus, if potassium chlorate is heated in the presence of a manganese dioxide catalyst: 2 KClO3 2 KCl + 3 O2 -8-