Lesson Guide
advertisement

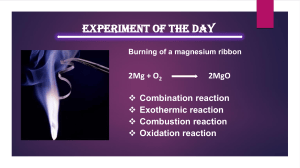
Lesson Guide CH. 24 – Chemical Reactions E. Q. – How do chemical reactions affect our everyday lives? Section 1 – Chemical Changes 1. What is a chemical reaction? Write the representation of how it proceeds from page 738. 2. What contribution to science did Lavoisier contribute concerning conservation of mass? 3. How did he do this and what did it lead to? 4. Write out the equation describing Figure 3 and then write out the chemical equation. 5. What are coefficients? 6. What makes the Statue of Liberty look green? Section 2 – Chemical Equations 1. Give two reasons for balancing equations. 2. Write a balanced equation for each of the following: a. Iron metal plus oxygen produces iron(II) oxide. b. Sodium metal plus water produces sodium hydroxide plus hydrogen gas. 3. What is understood if no coefficient is written before a formula in a chemical reaction? 4. See if you can balance the following: a. CaCo3 + HCl CO2 + CaCl2 + H2O Section 3 – Classifying Chemical Reactions 1. When does a combustion reaction take place? 2. What is a synthesis reaction? 3. What is occurring on the Titanic in Figure 9? 4. What is a decomposition reaction? 5. Classify the following: a. CaO(s) + H2O Ca(OH)2 (aq) b. Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s) c. NH4NO3(s) N2O(g) + 2H2O(g) 6. Rewrite the following equation using the smallest coefficient to give a balanced equation. a. 9Fe(s) + 12H2O(g) 3Fe3O4(s) + 12H2(g) Section 4 – Chemical Reactions and Energy 1. What forms does energy take when released or absorbed? 2. Who launched the first liquid fueled rocket and how high did it go? 3. What kind of fuel does the Lunar Module use? 4. Why is a log fire considered to be an exothermic reaction? 5. Why does dissolving Epsom salt in water cause it to become cold? 6. How do cold packs work?