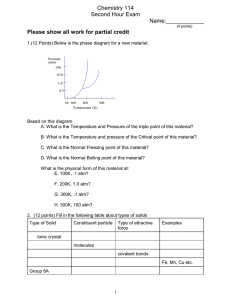
Honors Chemistry Name WS 7.3 Class ______ Date Complete each
advertisement

Honors Chemistry WS 7.3 Name _____________________________ Class _________ Date ________________ Complete each of the following sentences by filling in the appropriate word or phrase. 1. A cell is placed in a solution and bursts. This solution is a(n) ________________________ solution. 2. In ____________________ solutions, osmosis does not occur. 3. The temperature at which vapor pressures of solid and liquid phases are the same is the ___________________________ of a solution. 4. A cell is placed in a solution and shrinks. This solution is a(n) ______________________ solution. 5. The net flow of molecules from less concentrated to more concentrated solutions is ______________________________. 6. The temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure is the ____________________________ of a liquid. 7. What is the boiling point elevation of a solution containing 125 g of ethylene glycol (C 2H6O2) in 1200 g of water? Kb for water is 0.52C°/m 8. How many kilograms of ethylene glycol (C2H6O2) should be added to 25 kg of water to lower the freezing point by 45°C? K f for water is 1.86 C°/m. 9. A 6.0-g sample of an unknown compound is dissolved in 125 g of water. The solution’s freezing point is lowered to 1.38°C below the freezing point of pure water. What is the molar mass of the sample? K f for water is 1.86 C°/m. 10. A 15.0 g sample of an unknown compound is dissolved in 100.0 g of benzene (C6H6). The boiling point is raised 2.67°C above the boiling point of pure benzene. What is the molar mass of the sample? Kb for benzene is 2.67C°/m. 11. How many grams of benzoic acid (C7H6O2) must be dissolved in 78.1g of ethanol to raise the boiling point by 4.00 C? Kb for ethanol is 1.2 C°/m. 12. List the four colligative properties. 13. Explain why vapor pressure is reduced in a solution. 14. How is vapor pressure related to boiling point elevation and freezing point depression?