Chemistry 114 Second Hour Exam Name:____________ Please show all work for partial credit
advertisement

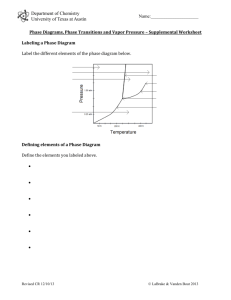
Chemistry 114 Second Hour Exam Name:____________ (4 points) Please show all work for partial credit 1.(12 Points) Below is the phase diagram for a new material. Based on this diagram A. What is the Temperature and Pressure of the triple point of this material? B. What is the Temperature and pressure of the Critical point of this material? C. What is the Normal Freezing point of this material? D. What is the Normal Boiling point of this material? What is the physical form of this material at: E. 100K, .1 atm? F. 200K, 1.0 atm? G. 300K, .1 atm? H. 300K, 100 atm? 2. (12 points) Fill in the following table about types of solids Type of Solid Constituent particle Type of attractive force Examples Ionic crystal molecules covalent bonds Fe, Mn, Cu etc. Group 8A 1 3. (12 points) The freezing point of nitrobenzene is 5.7oC and it has a freezing point depression constant of 6.87 K@m-1. How many grams of C6H4Cl2, (a non-ionic solute) will I have to dissolve in 1 kg of nitrobenzene to make the freezing point of the solution +1oC? 4A. (8 points) I am going to mix .2 moles of benzene with .8 moles of toluene. If the vapor pressure of pure benzene is 183 torr, and the vapor pressure of pure toluene is 59.2 torr, what is the vapor pressure of the solution? (Assume an ideal solution) 4B. (2 points) If the actual vapor pressure of this solution is higher that the number you calculated above, is this a POSITIVE or a NEGATIVE deviation from Raoult’s Law? (Circle one) 4C. (2 points)Based on the above deviation, are the solute-solvent interactions STRONGER or WEAKER than the respective solvent-solvent and solute-solute interactions (Circle one)? 2 5. I have determined the initial rate for the reaction A+B6C under three different conditions as shown in the data table below: [A] [B] 0.7 1 1 0.7 0.7 1.4 rate 0.018418087 0.0245 0.098 A. (3 points) Write the rate law equation for this reaction. B. (3 points) What is the order parameter for A? C. (3 points) What is the order parameter for B? D. (3 points) What is the overall rate constant for this reaction? 6. Rather than using the method of initial rates, I wish to determine the kinetics of a reaction by following the change in concentration over a 60 second time period. Below is a table of the data I obtained. Time (Seconds) Concentration (mM) 0 10 20 30 40 50 60 10 8.19E+00 6.70E+00 5.49E+00 4.49E+00 3.68E+00 3.01E+00 Since this method requires a graphing analysis, I have plotted this data three different ways as shown on the following page. 3 6A. (4 points) Is this a zero, first or second order reaction? B. (4 points) What is the rate constant for this reaction? C. (4 points) Use the information from parts A and B to write the Rate Law equation for this reaction. 7. (12 points) A reaction has a rate constant of 15 sec-1 at 25oC. What is the rate constant at 100oC, given that the activation energy is 15 kJ/mol? 4 8. (12 points) Define the following terms: Elementary step Intermediate species Steric factor Catalyst Half-life First Order Reaction 5