The Kinetic Theory, Pressure & Gas Laws
advertisement

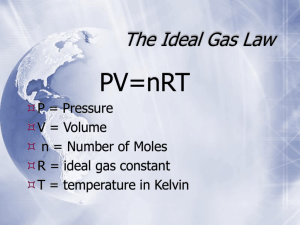
The Kinetic Theory, Pressure & Gas Laws GAS The four quantities needed to describe a gas: 1) number of particles 2) temperature 3) pressure 4) volume The Kinetic Theory The Kinetic Theory explains the effects of temperature & pressure on matter. • Idea that all particles of matter are constantly in motion 4 Assumptions: of gases for IDEAL GASES 1) All gases are composed of small particles and have no volume 2) These small particles move in continual, random, and rapid motion with no attraction or repulsion forces 3) All collisions are perfectly elastic 4) The average kinetic energy of the particles is directly proportional to its Kelvin temperature Real vs. Ideal Gases An ideal gas is one that follows the gas laws at all conditions of pressure and temperature and follows all the assumptions of the KMT An ideal gas does not really exist!!!!! Real gases can be liquefied and sometimes solidified The behavior of real gases under many conditions is similar to ideal gases. Each gas is different. Real gases behave like ideal gases at most conditions except for extremely high pressures and low temperatures Kinetic Theory con’t The physical behavior of a gas depends on its volume, temperature, and pressure. Temperature: Kelvin the average kinetic energy of the particles in an object ie. 25oC, O2 molecules 443 m/s; 1700 km/h; 1057 mi/h Absolute Zero: temperature at which all molecular motion should cease; lowest possible temperature -273.15oC or 0K Kinetic Theory con’t Temperature is not the total amount of thermal energy a substance has absorbed. Question 1: How is the average kinetic energy of water molecules affected when hot water from a kettle is poured into cups at the same temperature as the water? IT IS UNAFFECTED Kinetic Theory con’t Question 2: By what factor does the average kinetic energy of the molecules of a gas in an aerosol container increase when the temperature is raised from 300K (27oC) to 900K? The average kinetic energy triples Pressure Pressure: the force per unit area 1 object exerts on another SI Unit – Pascal (Pa) Gas Pressure: the pressure exerted by a gas it is created by collisions of gas particles with an object What happens to the pressure when you add more gas particles without changing its volume or temperature? Why? What happens to the pressure when you decrease the volume of the container for a fixed mass of a gas at a constant temperature? Why? What must happen to a gas’s temperature in order for its pressure to decrease? Why? Pressure Pascal: (Pa) • the SI unit for pressure • equivalent to 1N/m2 Millimeter of Mercury: (mmHg) pressure needed to support a column of mercury 1 mm high Pressure con’t Atmosphere: (atm) the pressure required to support 760 mm of mercury in a mercury barometer Torr: (torr) named after Evangelista Torricelli who invented the barometer Barometer: a closed-arm manometer used to measure pressure Pressure con’t 1 atm = 760 mmHg 1 atm = 101.325 kPa 760 mmHg = 101.325 kPa 1 torr = 1 mmHg 1 atm = 760 torr 1 atm = 760 mmHg = 760 torr = 101.325 kPa Question 3: Convert the following: a. 4.328 atm to kPa b. 328 kPa to mmHg c. 3290 Pa to atm 4.328 atm x 101.325 kPa 1.0 atm = 438.5 kPa 328 kPa x 760 mmHg 101.325 kPa = 2460 mmHg 3290 Pa x 1.0 atm 101325 Pa = 0.0325 atm manometer: a devise used to measure pressure of a gas Two Types of Manometers 1) closed-arm: used to measure the actual or “absolute” gas pressure *known as a barometer 2) open-arm manometer: one arm of the manometer is open to the atmospheric air to measure the gas pressure of a confined gas Avogadro’s Hypothesis Equal volumes of gases at the same temperature and pressure contain equal numbers of particles States of Matter 1) GASES: are independent of one another moving in straight lines until they collide with something that affects its direction and possibly its speed have no definite shape or volume gases assume the shape of the container 2) Liquids: have a definite volume but will take the shape of their container particles are attracted to one another but have enough energy to slide past each other no bond is formed between the particles 3) Solids: particles possess relatively fixed positions and vibrate around that fixed point attractive forces hold the particles extremely close but the particles of the solid are still traveling in straight paths between colliding with its exceedingly close neighboring particles the physical state of a substance at STP depends on the attractive forces verses the energy of the particles Ionic compounds: tend to be solids with strong electric charges Molecular compounds are attracted by van der Waals forces high molecular mass compounds tend to be solids nonpolar molecules of low molecular mass tend to be gases *the greater the mass and polarity, the more likely the compound will be a solid or liquid 4) Plasma: matter at temperatures greater than 5000oC causing a state where the matter is composed of electrons and positive ions magnetohydrodynamics Vaporization Vaporization: the conversion of a liquid to a gas or vapor below its boiling point (bp) Evaporation: the vaporization of an uncontained liquid Vapor Pressure: pressure created by a vapor in equilibrium with its liquid Vaporization con’t Boiling Point: (bp) temperature at which the vapor pressure of the liquid is equal to the external pressure Normal Boiling Point: boiling point for a substance at 1 atm * Boiling is a cooling process Vaporization con’t evaporation Liquid Vapor (gas) condensation Heat of Fusion: the additional amount of energy required to cause a phase change from a solid to liquid once a substance reaches its melting/freezing point Heat of Vaporization: the additional amount of energy required to cause a phase change from a gas to liquid once a substance reaches its boiling/condensation point Phase Changes Phase Diagram Triple Point: temperature and pressure where all three phases are at equilibrium Critical Point at the critical temperature and critical pressure of the substance, beyond this point the liquid and gas phases become indistinguishable JOHN DALTON DALTON’S LAW OF PARTIAL PRESSURES At a constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases PARTIAL PRESSURE: the pressure(contribution) each gas in a mixture makes to the total pressure of the mixture Ptotal = P1 + P2 + P3 … Example: Air contains oxygen, nitrogen, carbon dioxide, and trace amounts of other elements. What is the partial pressure of O2 at 1.00 atm if PN2 = 593.4 mmHg, PCO2 = 0.3 mmHg and Ptrace = 7.1 mmHg? Ptotal = PO2 + PCO2 + PN2 + Ptrace PO2 = Ptotal - (PCO2 + PN2 + Ptr) = 760mmHg - (593.4 mmHg + 0.3 mmHg + 7.1 mmHg) = 159 mmHg Your Turn: Determine the total pressure of a mixture of gases if the partial pressures of the gases are PO2 = 242.5 mmHg, PHe = 27.3 kPa, and PNe = 0.021 atm. PT = PO2 + PHe + PNe Look for unit agreement!!!! We will use mmHg as the common unit for our answer = 27.3 kPa X 760 mmHg = 205 mmHg 101.325 kPa = 0.021 atm X 760 mmHg = 16 mmHg 1 atm PT = 242.5 mmHg + 205 mmHg + 16 mmHg 464 mmHg Robert Boyle When the number of particles and temperature are constant, pressure and volume are inversely proportional. (opposite) P1 V1 = P2 V2 P1 = initial pressure V1 = initial volume P2 = final pressure V2 = final volume Example: A balloon is filled with 30.0 L of helium gas at 1.00 atm. What is the volume when the balloon rises to an altitude where the pressure is only 185.3 mmHg? Assume temperature remains constant. 30.0 L = V1 1.00 atm = P1 185.3 mmHg = P2 ? L = V2 Look for unit agreement 1.00 atm = 760 mmHg P1V1 = P2V2 Divide both sides by P2 V2 = P1V1 P2 V2 = (760 mmHg)(30.0 L) = 185.3 mmHg = 123 L Jacques Charles Charles’ Law states that the volume of a fixed mass of gas is directly proportional to its Kelvin temperature if the pressure is kept constant V1 = V2 T1 T2 *temperature must always be in Kelvin A 225 cm3 volume of gas is collected at 58.0oC. What volume, in liters, would this sample of gas occupy at standard temperature? V1 = 225 cm3 V2 = ? T1 = 58.0oC : 273.15 + 58.0oC = 331.2K T2 = 0.0oC : 273.15 + 0.0oC = 273.2K Charles’ Law V2 = V1T2 T1 = (225 cm3)(273.2 K) 331.2 K = 186 cm3 · 1 L = 1000 cm3 = 0.186 L Your Turn: A balloon, inflated in an air-conditioned room at 27.0oC, has a volume of 4.0 L. It is heated to a temperature of 57.3oC. What is the new volume of the balloon if the pressure remains constant? T1 = 27.0oC V1 = 4.0 L T2 = 57.3oC V2 = ???? Convert all temperatures to Kelvin V 1 = V2 T1 T2 V2 = V1 T2 T1 V2 = (4.0 L)(330.5K) 300.2 K = 4.4 L Joseph Louis Gay-Lussac Gay-Lussac’s Law the pressure of a given mass of gas is directly proportional to the Kelvin temperature if the volume is held constant P1 = P2 T1 T2 An acetylene gas cylinder has a pressure of 24350 mmHg at a temperature of 19.49oC. What would the internal pressure be if the temperature was increased to 100.0oC? Assume no change the volume of the cylinder. P1 = 24350 mmHg T1 = 19.49oC + 273.15 = 292.64 K P2 = X T2 = 100.00oC + 273.15 = 373.15K P1 T1 P2 P2 T2 = P2T1 T2 X = (24350 mmHg)(373.15 K) 294.64 K 24350 mmHg = X 292.64 K 373.15K = X = 31050 mmHg Your Turn: A gas has a pressure of 50.0 atm at 540 K. What will the temperature, in Celsius, be if the pressure is increased to 8330 kPa? P1 = 50.0 atm T1 = 540 K P2 = 8330 kPa T2 = X Unit agreement must be kept. Choose atm or kPa 50.0 atm x 101.325 kPa = 1.00 atm = 5070 kPa 5070 kPa = 8330 kPa 540 K X 890 K oC = 890 – 273 = 617oC Combined Gas Law Combines Boyle’s, Charles’ and Gay-Lussac’s laws together P1V1 = P2V2 T1 T2 all three laws can be derived from the combined gas law by removing the variable with a constant value The volume of a gas measured at 75.6 kPa pressure and 60.0oC is to be corrected to correspond to the volume it would occupy at STP. The measured volume of the gas is 10.0 cm3. P1 = 75.6 kPa V1 = 10.0 cm3 T1 = 60.0oC = 333.2 K P2 = 101.325 kPa T2 = 0.0oC = 273.2 K V2 = ????? cm3 P1V1 = P2V2 T1 T2 V2 = P1V1T2 P2 T1 V2 = (75.6 kPa)(273.2 K)(10.0 cm3) (101.325 kPa)(333.2 K) = 6.12 cm3 Your Turn: A cylinder of compressed oxygen gas has a volume of 30.0 L and 100.0 atm pressure at 300.0 K. The cylinder is cooled until the pressure is 5.00 atm. What is the new temperature, in Celsius, of the gas in the cylinder? V1 = 30.0 L P1 = 100.0 atm T1 = 27.0oC = 300.2 K V2 = 30.0 L P2 = 5.00 atm T2 = ???? K T2 = P2T1 P1 T2 = (5.00 atm)(300.2 K) 100.0 atm = 15.0 K 15.0 K = 273.15 + oC oC = - 258.2 IDEAL GAS LAW AVOGADRO’S HYPOTHESIS: gases at the same temperature, pressure and volume contain the same number of particles IDEAL GAS LAW PV = nRT PerVNeRT P = Pressure V = Volume in liters or dm3 n = moles R = ideal gas constant determined by the unit of pressure T = temperature in Kelvin R = 0.0821 atm·L mol·K = 8.31 kPa·L mol·K = 62.4 mmHg·L mol·K Ideal Gas Law A rigid steel cylinder with a volume of 20.0 L is filled with nitrogen gas to a final pressure of 200.0 atm at 27.0oC. How many moles of nitrogen gas does the cylinder contain? V = 20.0 L P = 200.0 atm T = 27.0oC + 273.2 = 300.2K n = ? mol CAN ONLY BE PV=nRT R=? R = 0.0821 atm·L/K·mol PV = nRT n = PV RT = (200.0 atm)(20.0 L) (0.0821 atm· L)(300.2 K) mol· K all units cancel except mol = 162 mol Your turn: Determine the pressure that 453.38 g of oxygen gas would exert if it is put into a container with a volume of 25.0 mL and a temperature of 17.4oC. m = 453.38 g O2 V = 25.0 mL T = 17.4oC P=? Must be PV=nRT LOOK FOR UNIT AGREEMENT n = 453.38 g O2 X 1 mol O2 = 14.2 mol 31.9988 g O2 V = 25.0 mL = 0.0250 L T = 17.4oC + 273.2 = 290.6 K P = ? atm R = 0.0821 L atm/K mol P = nRT V P = (14.2 mol)(0.0821 L atm/K mol)(290.6K) 0.0250 L P = 13600 atm Graham’s Law of Effusion Diffusion: the tendency of atoms, ions or molecules to move toward areas of lower concentration until there is a uniform composition Effusion: occurs as a gas escapes through a tiny hole in a container Graham’s Law the rate of effusion of a gas in inversely proportional to the square root of its molar mass RateA = RateB molar mass B molar mass A Example Which gas effuses faster, carbon dioxide or neon? By how much faster? Remember, lighter moves faster CO2 = 44.0098 g/mol Ne = 20.179 g/mol Ne is faster because its molar mass is smaller RateNe = RateCO2 = 44.0098 g/mol 20.179 g/mol 1.477 neon gas is 1.477 times faster than carbon dioxide Example 2 Determine the molar mass for a gas that is 0.6372 times as fast as oxygen gas. Rateunknown = Rateoxygen Molar mass oxygen molar mass unknown Rateunknown = 0.6372 Rateoxygen 2 0.6372 = 31.9988 g/mol X 0.4060 = 31.9988 g/mol X X = 78.81 g/mol 2 14.83 g zinc reacts with an excess of hydrochloric acid, HCl, in the production of hydrogen gas. Calculate the pressure, in kPa, of the gas collected if it has a volume of 2.150 L at a temperature of 25.0oC. Assume 100% yield. Zn + 2 HCl → ZnCl2 + H2 mass Zn = 14.83 g PH2 = ? kPa VH2 = 2.150 L TH2 = 25.0oC + 273.2 = 298.2K All means Gas Law Stoichiometry Zn + 2HCl → ZnCl2 + H2 14.83 g Zn X 1mol Zn X 1 mol H2 = 65.39 g Zn 1 mol Zn = 0.2268 mol H2 P = nRT V P = (0.2268 mol)(8.31 kPa·L/K·mol)(298.2 K) 2.150 L P = 261.4 kPa Acetylene, C2H2, is a gas used in cutting metal. If 1.70 x 103 g of acetylene gas undergoes a complete combustion reaction, calculate the mass of oxygen gas that would be required to react with all the acetylene gas. Second, calculate the pressure of the oxygen gas if the container the acetylene is in has a volume of 55.00 L and a temperature of 84.56oC. 2 C2H2 + 5 O2 → 4 CO2 + 2 H2O 1.70 x 103 g C2H2 X 1 mol C2H2 X 5 mol O2 26.0379 g C2H2 2 mol C2H2 163 mol O2 X 31.998 g O2 1 mol O2 5220 g O2 = = n = 163 mol V = 55.00 L T = 84.56 + 273.15 = 357.71 K P = (163 mol)(0.0821 L· atm/ K·mol)(357.71 K) 55.00 L 87.0 atm Calculate the mass of nitrogen gas required to react hydrogen gas with a pressure of 356.33 kPa, a temperature of 288.3 K and a volume of 377.4 mL in the production of ammonia. N2 + 3 H2 → 2 NH3 nH2 = PV RT (356.33 kPa)(0.3774 L) (8.31 kPa·L)(288.3 K) K·mol = 0.05613 mol H2 = 0.05613 mol H2 X 1 mol N2 3 mol H2 = 0.5241 g N2 X 28.0134 g N2 1 mol N2 =