Warm-ups 2nd marking period
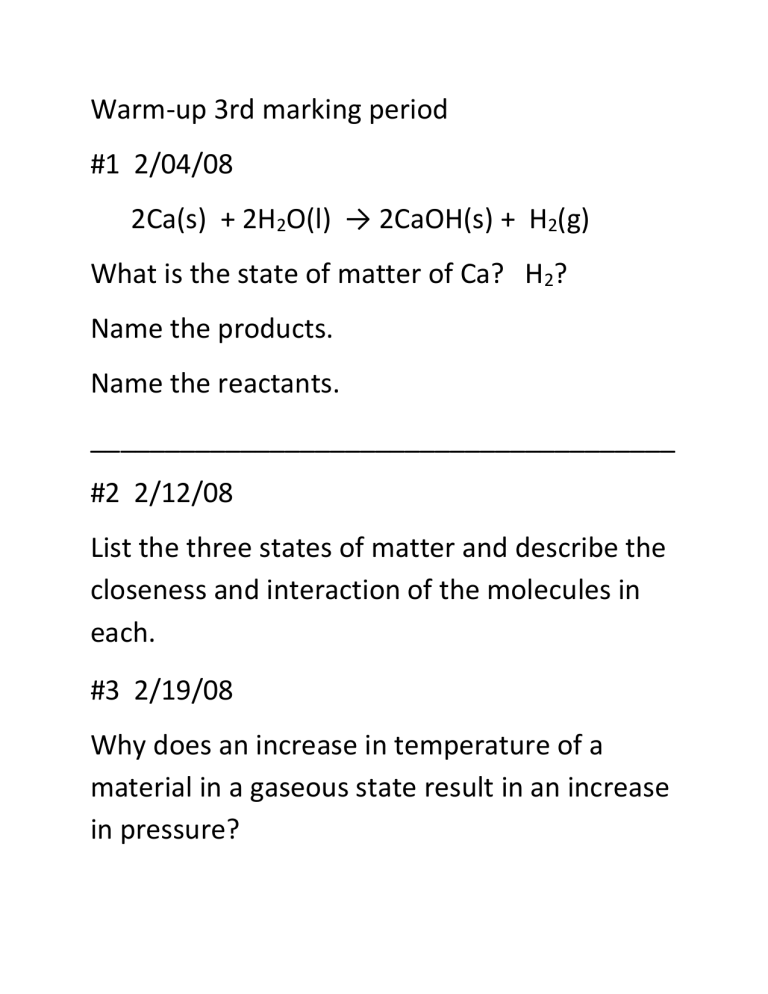
Warm-up 3rd marking period
#1 2/04/08
2Ca(s) + 2H
2
O(l) → 2CaOH(s) + H
2
(g)
What is the state of matter of Ca? H
2
?
Name the products.
Name the reactants.
_______________________________________
#2 2/12/08
List the three states of matter and describe the closeness and interaction of the molecules in each.
#3 2/19/08
Why does an increase in temperature of a material in a gaseous state result in an increase in pressure?
#4 2/20/08
Write the definition of an amorphous material, a liquid crystal and plasma.
#5 2/21/08
If you blow up a balloon with hot air and then put it in the freezer, what will happen to the balloon?
_______________________________________
#6 2/26/08
Convert the following temperatures a.
100 o C to K b. 228K to o C b.
478 o C to K d. 2K to o C
_____________________________________________
#7 2/29/08
What is the difference between Brownian motion and diffusion?
#8 3/05/08
It takes 4000joules of heat to vaporize water at
100 o C. The heat of vaporization is 2260J/g.
How much water is vaporized?
#9 3/11/08
Convert the following:
1.) 1325 pennies to quarters
2.) 4.8 ft to cm
3.) 8 lb to g
#10 3/12/08
1 mile = 1.6 km 1 atm = 760 mmHg
Convert the following:
1.) 27 hm to dm
2.) 105 km to miles
3.) 3 atm to mmHg
#11 3/24/08
1 gallon = 3.8 L 1 mile = 1.6 km
Convert the following:
1.) 250 cm to hm
2.) 4.5 gallons/hr to liters/min.
3.) 105 km/hr to miles/hr
#12 3/25/08
1 in. = 2.54cm 1 mile = 1.6 km
Convert the following:
1.) 22 in. to mm
2.) 10 m to cm
3.) 72 miles/hr to km/min.
#13 3/26/08
Convert the following:
1.) 43.8 lb/in 2 to atm
2.) 845 mm Hg to kPa
3.) 680 in. Hg to mm Hg
#14 3/31/08 Warm-ups due 4/4/08.
You need 15 warm-ups.
Fill in what you know for the problem below.
The pressure of a piston is 3 atm and the volume is
1.2 liters. If temperature remains constant and the pressure is reduced to 2 atm, what is the new volume?
P
1
= V
1
= P
2
= V
2
=
#15 4/01/08
The volume of a capped syringe is 5ml. The pressure is 101.3kPa. The pressure is increased to 132 kPa. What is the new volume?
_______________________________________
#16 4/02/08
The volume of a balloon is 50ml and has a temperature of 30 o C. If the temperature is reduced to 10 o C, what is the new volume?











