Topic 3 Alkanes
advertisement

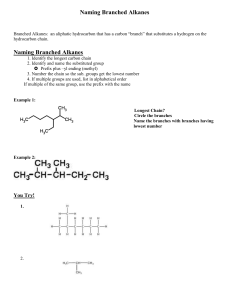
Alkanes Carbon has 4 bonding electrons Carbon can bond to itself in chains or rings Carbon can form single, double and triple bonds Carbon can form isomers (same formula, different names, characteristics and properties) Organic Compounds • Carbon and Hydrogen (bonded together) • Excluding OXIDES, CARBIDES, CARBONATES, CYANIDES Complete structural diagram • i.e. 3-methyl heptane Condensed Structural Diagram • i.e. 3 methyl heptane Line Structural Diagram • Aka Skeleton diagram • i.e. 3-methyl heptane ALKANES • Hydrocarbon compounds • All carbons are SATURATED (meaning all single bonds) • Acyclic (meaning no loops) CnH2n+2 • i.e. C7H16 IUPAC Naming • the root is names using the longest continuous chain of carbon atoms and applying the standard greek name • the suffix “ane” is added to the appropriate numbering Prefix Meth Eth Prop But Pent Hex Hept Oct Non Dec # of Carbons 1 2 3 4 5 6 7 8 9 10 • every side chain is named by subtracting the “ane” and adding “yl” suffix • each side chain is numbered by its distance from the first carbon in the chain (so to have the lowest numbers) Example • Name the following alkane Example 2 • Name the following alkane CH3CH2CHCH2CH3 CH3 Example 3 • Name the following alkane Example 4, 5, 6, 7 • 3,3,4,5-tetramethyl decane • 3-ethyl 5-methyl octane • 4-butyl 3-ethyl 2,6-dimethyl nonane • 3-ethyl 2,3,3-trimethyl heptane Commercial Importance of Alkanes • Natural gas = mainly methane (also includes ethane, propane, butane and pentane) • Natural gas is heated in a fractional distillation town to remove H2S • Petroleum = variety of alkanes separated by fractional distillation • Fuel, wax, lubricants, asphalt, tar and sulfur all come from petroleum • Pharmaceuticals, fertilizers, pesticides and plastics are manufactured from petroleum Properties of Alkanes • Non polar molecules • as the length of the chain increases, the sum of the intermolecular forces increases • shape and size affect the boiling point • Increase the branching decreases the boiling point • Increasing the number of carbons increases the boiling point