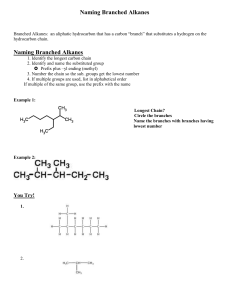
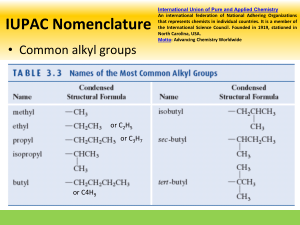
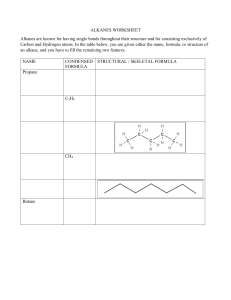
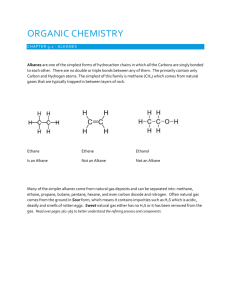
Naming Branched Alkanes Branched Alkanes: an aliphatic hydrocarbon that has a carbon “branch” that substitutes a hydrogen on the hydrocarbon chain. Naming Branched Alkanes 1. Identify the longest carbon chain 2. Identify and name the substituted group Prefix plus –yl ending (methyl) 3. Number the chain so the sub. groups get the lowest number 4. If multiple groups are used, list in alphabetical order If multiple of the same group, use the prefix with the name Example 1: Longest Chain? Circle the branches Name the branches with branches having lowest number Example 2: You Try! 1. 2. Halogenated Alkanes Similar to naming branched alkanes, you need to include a number, indicating which carbon the halogen is bonded to. To name the halogen, use fluoro, chloro, bromo, or iodo. Practice: 2-chloro pentane 2 bromo, 3 fluoro heptane 2,3 dichloro pentane Cyclo-hydrocarbons An organic compound consisting of carbons that are singley bonded in a ring. Naming: same as before, only include the prefix cyclo Name the following branched alkanes: Drawing Branched Alkanes Drawing Branched Alkanes: Draw the longest chain first Place alkyl groups on the designated carbons Place remaining spaces with hydrogens, if structural Draw the structural formula or skeletal formula for the following molecules. Remember the following: Carbons on the end of a chain are attached to three hydrogens Carbons in the middle of a chain are attached to two hydrogens Carbons that have one branch attached are also attached to one hydrogen Carbons that have two branches attached are not attached to any hydrogens Examples 1: 2,3 dimethyl- heptane Example 2: 4 methyl , 3 propyl- octane 4-ethyl-octane 3,3-dimethyl-pentane 3-ethyl-pentane 3-ethyl-2methyl-heptane 2,2,3-trimethyl-butane 3 bromo, 2 fluoro, 3 methyl heptane