Alkane Physical Properties: Density & Boiling Point Worksheet
advertisement

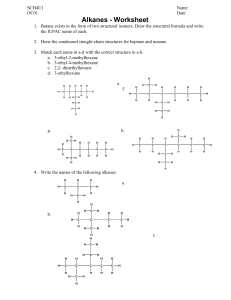
Alkanes: Physical Properties Your task 1. The table below shows some physical data (density and boiling point) for some alkanes. Complete the table. Alkane Density (g cm-3) Tb (K) 0.466 0.572 0.585 0.557 0.601 0.591 0.620 0.626 0.653 0.660 0.679 0.684 0.698 0.703 0.718 0.730 0.740 0.749 0.775 0.789 109.1 184.5 231.0 261.4 272.6 282.6 301.0 309.2 333.4 342.1 363.1 371.5 390.7 398.8 423.9 447.2 469.1 489.4 560.1 616.9 methane ethane propane 2-methylpropane butane 2,2-dimethylpropane 2-methylbutane pentane 2-methylpentane hexane 2-methylhexane heptane 2-methylheptane octane nonane decane undecane dodecane hexadecane eicosane Number of carbon atoms 2. Plot a graph (or graphs) to show the way in which each physical property changes with increasing number of carbon atoms. The number of carbon atoms should be on the horizontal axis. Label any unusual points on your graph(s). Now answer the following questions as fully as you can using information from your graph or graphs. 3. How does the size of alkane molecule affect the density and the boiling point? 4. How does the shape of the alkane molecule affect these physical properties? Consider in turn: • • • straight chain alkanes; single branch alkanes; double branch alkanes. Copyright © 2003 Nigel Saunders N-ch3-04