ATOMIC SPECTRA
advertisement

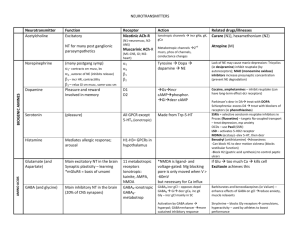
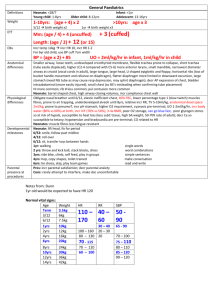
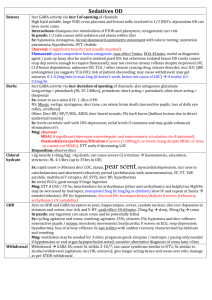
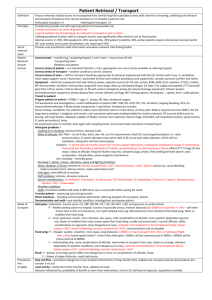
PERIODIC PROPERTIES Nuclear Charge (z) Effective N C (Zeff) Ionization E & Affinity Trends on P. Table Isoelectronic Series Properties of Elements EFFECTIVE NUCLEAR CHARGE Zeff nucleus & e- density of a many-e- atom strength of attraction: 1) as magnitude of charges 2) distance from nucleus 3) L to R, slight down col nucleus: 12 p+ inner core [Ne]: 10 evalence, 3s: 2 e- 12 – 10 = 2+ Shielding Penetration e- interaction; repulsion of e- consider orbital shapes decr full nuclear charge, Zeff i.e. 2p closer to nucleus, but 2s probable distr w/i 1s, therefore, 2s penetrates closer to nucleus Lower l value, more these e- penetrate Sublevel E order: s<p<d<f Effect of Nuclear Charge + nucleus <==> e- attraction charge, then attraction pulls orbitals closer to nucleus more stable, more E required to remove e- TREND -- Main Groups ATOMIC SIZE Incr L to R & down col 2 influences 1. es in E-levels as E-levels, n, increase then also radius 2. Nucleus’ “+” charge, Zeff as nucleus increases “+” chrg, draws E-levels in closer to nucleus, thus, decr radius IONS 1. 1. anions > cations more “+” cation is smaller more “-” anion is larger 2. same charge cation; incr down col decr L to R 1A > 2A > 3A SIZE Fig 7.6 pg 263 nonbonding radius: closest dist separating nucleus of colliding atoms bonding radius: 1) attractive interaction bet atoms 2) atoms closer together than non-, 3) is dist that separates nuclei of bonded atoms bonding radius < nonbonding radius trend bonding radius pg. 263 ion size depends on: cation radius < parent element anion radius > parent element ISOELECTRONIC SERIES group of ions w/ same # elist ions incr atomic #; Zeff incr; results in ion radius decr ex. pg 266 O-2 – F-1 – Na+1 – Mg+2 – Al+3 10 eeffect: incr Z as decr radius Practice Problems 1)What is the As – I bond length in AsI3? 2)Arrange in incr radius: F, P, S, As 3)What neutral element is isoelectronic to Al+3, Ti+4, Br-1? [As 1.19] + [I 1.33] = 2.52 Å F < S < P < As Ne, Ar, Kr IONIZATION ENERGY Min E required to remove e- from grd state to form cation Li + E --- Li+1 + e- Ca + E --- Ca+1 + eCa+1 + E -- Ca+2 + eI1 < I2 < I3 < etc notice balance of charges as I incr, more diff to remove e**drastic incr E remove inner core e- Ionization Values Table 7.2 pg. 268 Trends 1st Ionization E Values Fig. 7.10 pg. 270 np4 e- easier to remove than np3 E required to remove eIEvalence shell e- < IEinner core eLi Z= 3 1s22s1 IE1= 520 kJ valence e- IE2= 7300 kJ 1st inner core e- N Z=7 1s22s2p3 valence shell e- IE1 --> IE5 1402 kJ ---> 9440 kJ inner core e- IE6 ---> IE7 53270 kJ ---> 64360 kJ Thus w/ the greater E required to remove inner core, those e- not involved in chem rxns s TREND, Incr across I1 row, decr down col smaller atom I1 > larger atom I1 p incr to ½-fill (3 e-), decr as remove from 1 pair, then incr from s2 to p1, e- must enter empty subshell p3 to p4; repulsion of pair, each p3 is single (ml) Fig 7.12 pg 272 ELECTRON AFFINITY E by addition of e- to grd state to form anion Br + e- --- Br-1 + E O + e- --- O-1 + E O-1 + e- --- O-2 + E -kJ/mol: indicates add e- is exo +kJ/mol: E released when e- added e- AFFINITY (EA) EA: E (kJ) required (released) to add 1 mol e- to 1 mol gaseous subst I (g) + e- -------> I-1(g) + E E = EA1 < 0 EA2= “+” value EA2 > EA1 Net Effect • trend not as consistent as w/ IE • typically decr down a col • typically incr L--->R across row usually stronger attraction for e- > neg affinity value + value, e- not attached, ion unstable p: incr across row; drop @ p3 p 1 < p2 < p4 < p5 < p6 p2 > p3 < p4 METALS - NONMETAL - METALLOIDS ReVieW pg. 273 metals shiny luster malleable, ductile conduct heat, elec solid @ room temp form + cations metal oxides form bases form alloys w/ metals nonmetal metalloid no luster have some metallic not malleable, ductile can conduct poor conductors -semiconductor solids usually brittle form - anions w/ metals oxides form acids form molecules w/ non Incseasing Metallic Behavior Fig 7.13 pg.274 GROUP TRENDS IA - Alkali (ashes) soft metals; metallic luster low densities; low M.P. down col I1 decr, radius incr, react incr exist as cmpds only alkali + H2 -------> Hyrdide LiH NaH alkali + H2O ----> Base LiOH NaOH Ho = - EXO form +1 cations IIA - Alkaline solids compare to IA: harder, more dense, higher M.P., reactive but less, I2A > I1A down col react incr form +2 cation NONMETALS hYDroGeN nonmetal, no group memeber metallic under pressure no shielding elarge I1 - 1300 as Li 500 H2 + non ----> Ho = - EXO more in common w/ 7A H2 + metal ----> Hydride NaH CaH2 OXYgen - 6A only gas in group, all solids O2 ----> O3 Ho = + ENDO O3 < stable OXYgen - 6A down col M.P. & B.P. incr diatomic molecules high EA values, high to gain every soluble in H2O ----> hydro-hal-ic acid HF HCl HBr HI Noble Gases - 8A (inert; unreact) nonmetals, monoatomic complete filled s & p (s2p6) LARGE I1 ACID - BASE Metals - Main ME + O2 ----> OXIDE 2 Ca + O2 ----> 2 CaO BEHAVIOR Nonmetals Ionic ME oxide + H2O ----> BASE CaO + H20 ----> Ca(OH)2 NM + O2 ----> OXIDE S + O2 ----> SO2 Covalent NM oxide + H2O ----> ACID SO2 + H20 ----> H2SO3 Metalloids form amphorteric subst.