Chemical Nomenclature
advertisement

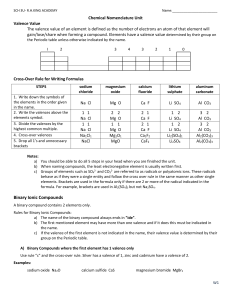
Chemical Nomenclature Binary Compounds • Simplest compounds; made up of two kinds of atoms or monoatomic ions (for an ionic compound) • For binary ionic compounds: – The positive metal is first, followed by the negative non-metal – Name of metal is stated in full and non-metal has an –ide suffix • Write chemical formula for aluminum chloride: Al 3 Al1Cl3 Cl 1 AlCl3 • Name the following chemical: Li2O lithium oxide Try: a) b) c) d) e) f) BaS magnesium nitride sodium bromide Al2O3 calcium chloride BN a) b) c) d) e) f) barium sulfide Mg3N2 NaBr aluminum oxide CaCl2 boron nitride • Some metals have more than one charge. They are known to be multivalent. Metal Ion IUPAC Name iron Fe2+, Fe3+ iron(II), iron(III) copper Cu+, Cu2+ copper(I), copper(II) tin Sn2+, Sn4+ tin(II), tin(IV) lead Pb2+, Pb4+ lead(II), lead(IV) cobalt Co2+, Co4+ cobalt(II), cobalt(IV) gold Au+, Au2+ gold(I), gold(II) mercury Hg+, Hg2+ mercury(I), mercury(II) Write the IUPAC name for: a) SnCl4 a)tin(IV) chloride b)gold(I) bromide b) AuBr c)lead(IV) oxide c) PbO2 Write the chemical formula for: a) mercury(II) oxide a)HgO b) copper(II) sulfide b)CuS c) iron(III) chloride c)FeCl3 Polyatomic Ions • Ions that have more than 2 atoms present • Eg. ClO3-, SO42-, PO43Know the following 10 for test: acetate (CH3COO-) nitrate (NO3-) ammonium (NH4+) nitrite (NO2-) carbonate (CO32-) phosphate (PO43-) chlorate (ClO3-) sulfate (SO42-) hydroxide (OH-) sulfite (SO32-) Try: a)hydrogen chlorate b)PbSO4 c)iron(III) nitrate d)magnesium sulfite e)Ca3(PO4)2 f) NaNO2 g)lithium carbonate a) HClO3 b) lead(II) sulfate c) Fe(NO3)3 d) MgSO3 e) calcium phosphate f) sodium nitrite g) Li2CO3 Molecular Compounds • Binary compound consisting of two non-metals • Use IUPAC prefixes for naming (eg. mono, di, tri, etc) p. 97 – Do not use “mono” for first part of binary compound name, only for 2nd part! PCl3 phosphorus trichloride (no “mono” for phosphorus) P2S5 diphosphorus pentasulfide Try: a) sulfur trioxide b) P4O7 c) dinitrogen trioxide d) NCl3 e) CCl4 a) SO3 b) tetraphosphorus heptoxide c) N2O3 d) nitrogen trichloride e) carbon tetrachloride Acids • All acids start with H (e.g. HCl, H2SO4) • TWO acids types exist: a) binary acids and b) oxyacids – Binary: H + non-metal. E.g. HCl – Oxyacids: H + polyatomic ion. E.g. H2SO4 • Each have different naming rules. Naming Binary Acids – depends on the state of the acid • If it’s NOT in aqueous state [eg. (g)] – hydrogen + non-metal hydrogen chloride – HCl(g) • If it is aqueous (aq): – hydro + non-metal root + ic + acid – HCl(aq) hydrochloric acid Naming Oxyacids – does not depend on the state 1) Name the polyatomic ion 2) Replace -ate with -ic, -ite with -ous 3) Change non-metal root for pronunciation 4) add “acid” to the name Eg. H2SO4 H2SO3 sulfuric acid sulfurous acid Try: a) b) c) d) e) hydrobromic acid HNO3 nitrous acid HF (g) H3PO4 a) b) c) d) e) HBr (aq) nitric acid HNO2 hydrogen fluoride phosphoric acid Bases For this course, we will limit ourselves to the hydroxides (OH-): Formula IUPAC Name NaOH sodium hydroxide Ba(OH)2 barium hydroxide KOH potassium hydroxide Ca(OH) 2 calcium hydroxide Etc...