Formula Name of Salt - TopekaWest
advertisement

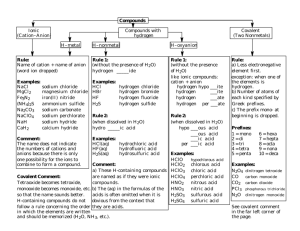
Nomenclature Worksheet I. Binary Compounds A binary compound is one containing only two elements 1. The more metallic element is named first, followed by the more non-metallic element. 2. The name of the non-metallic element carries the suffix ide. i. Examples: CaO = calcium oxide CaCl2 = calcium chloride ii. CaS = calcium sulfide CaH2 = calcium hydride 3. The ammonium ion, NH4+; the hydroxide ion, OH-; and the cyanide ion, CN-; are treated as single elements. NH4OH = ammonium hydroxide NaCN = sodium cyanide 4. If the metallic element is capable of more than one valence the roman numeral distinguishes which ion is used. FeCl3 = iron (III) chloride CuO = copper (II) oxide, FeCl2 = iron (III)chloride, Cu2O = copper(I)oxide Name the following ionic compounds- use your text or ion chart as needed. 1. CaO _________________________ 13. CaCl2____________________________ 2. NaCl _________________________ 14. Cu2S ____________________________ 3. NaCN _________________________ 15. KH ____________________________ 4. SbBr3 __________________________ 16. NH4CN _________________________ 5. PbO __________________________ 17. Hg2F2 __________________________ 6. MgS __________________________ 18. HgO ___________________________ 7. NH4Br _________________________ 19. FeCl2 ___________________________ 8. AgCN __________________________ 20. Ag2O ___________________________ 9. MnBr2__________________________ 21. AlBr3 ___________________________ 10. CuO ___________________________ 22. Fe(OH)3 _________________________ 11. ZnI2 ___________________________ 23. Ba(OH)2_________________________ 12. K3N ___________________________ 24. PbBr2 ___________________________ Covalent Compounds When naming compounds of a given pair of elements exist, they are best named by using the prefixes mono, di, tri, tetra, penta, hexa, hepta, and octa to indicate the number of atoms involved. The prefix mono is usually omitted. N2O = dinitrogen monoxide or dinitrogen oxide NO = nitrogen oxide N2O3 = dinitrogen trioxide, often called nitrogen trioxide NO2 = nitrogen dioxide N2O4 = dinitrogen tetraoxide, ofen called nitrogen tetraoxide N2O5 = dinitrogen pentoxide, often called nitrogen pentoxide Name the following compounds: 25. CCI4____________________________ 39. ICl3 ________________________ 26. PCl5 ___________________________ 40. H2S3 ________________________ 27. SI6 ____________________________ 41. N2O3 ___________________________________ 28. I2O4___________________________ 42. I2O9 ________________________ 29. BrO2__________________________ 43. SO3 _________________________ 30. N2F4 _________________________ 44. NI3 __________________________ 31. N2O4___________________________ 45. Br2O_________________________ 32. P3N5 __________________________ 46. BN2__________________________ 33. SF6___________________________ 47. CS2__________________________ 34. CO __________________________ 48. F2O_____________________________________ 35. NF3 __________________________ 49. P4S7_________________________ 36. SiO2__________________________ 50. CO2 _________________________ 37. CBr4 _________________________ 51. IF7 __________________________ 38. SO2 __________________________ 52. P2O5 _________________________ Formula Writing Practice Worksheet #2 Name: Correct the following formula, if necessary, and name the compounds. Correct formula Name of compound 1. Cd(CN) __________________ _________________________ 2. BN __________________ _________________________ 3. Ca(OH) __________________ _________________________ 4. RaCl __________________ _________________________ 5. AgS __________________ _________________________ 6. AlO __________________ _________________________ 7. KS __________________ _________________________ 8. CuBr __________________ _________________________ 9. Zn(OH) __________________ _________________________ 10. (NH4)S __________________ _________________________ 11. Mg3N2 __________________ _________________________ 12. NaH __________________ _________________________ 13. Ba(CN) __________________ _________________________ 14. AgO __________________ _________________________ 15. SnBr2 __________________ _________________________ 16. CuF2 __________________ _________________________ 17. SnCl __________________ _________________________ 18. AlBr __________________ _________________________ 19. Cu2O __________________ _________________________ 20. FeCl __________________ _________________________ 21. Ba(OH) __________________ _________________________ 22. Al(OH)3 __________________ _________________________ 23. (NH4)(OH) __________________ 24. AlN ____________________ _________________________ _________________________ 25. SnO2 ______________________ _________________________ 26. RaO _____________________ _________________________ 27. SiCl ______________________ _________________________ 28. Zn(C2H3O2)_____________________ _________________________ 29. MgI ______________________ _________________________ 30. CuO ______________________ _________________________ 31. BH ______________________ _________________________ 32. ZnCl ______________________ _________________________ 33. BeCl ______________________ _________________________ 34. CCl4 ______________________ _________________________ 35. N2O ______________________ _________________________ 36. PtBr _____________________ _________________________ 37. NBr _______________________ _________________________ 38. GeBr4 _______________________ _________________________ 39. K2(CO3) ______________________ _________________________ 40. AsO _______________________ _________________________ 41. P2O5 ________________________ _________________________ 42. NaS _______________________ _________________________ 43. KN ________________________ _________________________ 44. SI6 ______________________ _________________________ 45. Hg2O3 ______________________ _________________________ 46. ScO5 ____________________ _________________________ 47. (NH4)(CN)_____________________ _________________________ 48. MgC _______________________ _________________________ 49. BC _______________________ _________________________ 50. SiN _______________________ ___________________________ II. Acid Nomenclature A. Binary acids may be named as binary compounds or by the following rules: 1. Use either the stem or the full name of the nonmetallic element 2. Add the prefix hydro. 3. Add the suffix ic. H2S = Hydrosulfuric acid HCl = Hydrocholoric acid Name the following acids: 1. HBr _____________________________ ___________________________ 3. H2Se 2. HI ______________________________ ___________________________ 4. H2Te B. Ternary acids are composed of three elements: hydrogen, a nonmetal, and oxygen 1. The most common acid formed by these three elements is named by adding the suffix ic to the stem of the non-metal. H2SO4 = Sulfuric acid H2SeO4 = Selenic acid HClO3 = Chloric acid HBrO3 = Bromic acid HNO3 = Nitric acid H3PO4 = Phosphoric acid 2. The acid containing one less oxygen atom than the ic acid is named by adding the suffix ous to the stem of the non-metal. HClO2 = Chlorous acid 3. The acid containing one less oxygen atom than the ous acid (or two less oxygen atoms than the ic acid) is named by adding the prefix hypo and the suffix ous to the stem. HClO = Hypochlorous acid 4. The acid containing one more oxygen atom than the ic acid is named by adding the prefix per and the suffix ic to the stem. HClO4 = Perchloric acid Supply the Missing Name or Formula for the acids listed below 1._____________________Hydrofluoric acid 20. HNO3 ___________________________ 2. HIO3___________________________ 21. H3PO3__________________________ 3. H2Se___________________________ 22. HBrO __________________________ 4. HNO2__________________________ 23. HClO __________________________ 5. H2Te___________________________ 24. H2SeO4_________________________ 6. HI ____________________________ 25. ___________________Arsenous acid 7. H3AsO2________________________ 26. HBrO3 ________________________________________ 8. H2SeO3_________________________ 27. H2SO3 __________________________ 9. HAtO3__________________________ 28. ______________________Bromic acid 10. ______________________Carbonic acid 29. HCl_____________________________ 11. HAtO_________________________ 30. HMnO4_________________________ 12. H3AsO4________________________ acid 31. ___________________Hypochlorous 13. H3PO4_________________________ 32. _____________________Selenic acid 14. _____________________Hydrochloric acid 33. HBr ________________________ 15. H3AsO3________________________ 34. H2S__________________________ 16. H2CO3_________________________ 35. HIO _________________________ 17. H2SO4__________________________ 36. ____________________Nitrous acid 18. H3PO2__________________________ 37. HF _____________________________ 19. HClO4__________________________ 38. _________________Hydrosulfuric acid III. Bases Bases are named as binary compounds with the hydroxide ion as the nonmetal. IV. Salts A. Salts of binary acids are named as binary compounds. B. Salts of ternary acids are named as binary compounds except that the place of the non-metal is taken by the name of the acid radical, derived as follows: 1. Salts derived from ic acids – substitute the suffix ate. Chloric acid HClO3 Sodium chlorate NaClO3 2. Salts derived from ous acids – substitute the suffix ite. Chlorous aicd HClO2 Sodium chlorite NaClO2 3. Salts derived from hypo…ous acids – retain the prefix hypo but substitute the suffix ite. Hypochlorous acid HClO Sodium hypochlorite NaClO 4. Salts derived from per…ic acids – retain the prefix per but substitute the suffix ate. Perchloric acid HClO4 Sodium perchlorate NaClO4 Complete the following table Formula of acid Name of acid Formula of Name of potassium salt potassium salt 1. H2SO4 ____________________ ____________________ __________________ 2. HClO ____________________ ____________________________________ 3. H3PO4 ____________________ ______________________________________ 4. H2CO3 ____________________ _______________________________________ 5. HClO4 _________________________________________________________ 6. H3AsO4___________________ _______________________________________ 7. HCl ___________________ _______________________________________ 8. HNO3 ___________________ _______________________________________ 9. H2BrO3___________________ _______________________________________ 10.HBrO3 ___________________ _______________________________________ Formula of Name of acid Acid Formula of Name of Barium Salt Barium Salt 11._________ Selenic acid _________ _______________ 12._________ Chlorous acid _________ _______________ 13._________ Hydrobromic acid _________ _______________ 14._________ Nitric acid _________ _______________ 15. _________ Phosphorous acid _________ _______________ 16. _________ Carbonic acid _________ _______________ Formula of Name of acid Formula of Name of Aluminum salt Aluminum salt Acid 17._________ ________________ __________ Aluminum nitrite 18._________ ________________ __________ Aluminum sulfite 19. _________ ________________ __________ Aluminum arsenate 20. _________ ________________ __________ Aluminum hypochlorite 21._________ ________________ ___________ Aluminum phosphate 22._________ ________________ ___________ Aluminum chlorate Formula Name of Salt Of Salt 23._________ ________________ MgSO4 ___________________ 24. _________ ________________ Cd(BrO3) ___________________ 25. _________ ________________ Cs2SO4 ___________________ 26. _________ ________________ Fe(NO3)2 ___________________ 27. _________ ________________ Ag3PO4 ____________________ 28. _________ ________________ CuS ____________________ 29. _________ ________________ NH4Br ____________________ 30. _________ ________________ BaCO3 ____________________ 31. _________ ________________ Fe2(SeO4)3 ____________________ 32. _________ ________________ SbCl3 ____________________ Formula of Acid Name of Acid Formula of Salt Name of Salt 33.________ _________________ BaSeO4 _______________ 34.________ _________________ MgCr2O7 _______________ 35. ________ _________________ ZnS _______________ 36. ________ _________________ NaMnO4 _______________ 37. ________ _________________ MgSO3 _______________ 38. ________ _________________ ZnCO3 _______________ 39. ________ _________________ LiClO4 _______________ 40. ________ _________________ Be3(PO4)2 _______________ 41. ________ _________________ CuSeO3 _______________ 42. ________ _________________ Cd(ClO3)2 _______________ 43. ________ _________________ AgC2H3O2 _______________ 44. ________ _________________ KNO3 _______________ 45. ________ _________________ SrCl2 _______________ 46. ________ _________________ LiClO4 _______________ 47. ________ _________________ Al(ClO)3 _______________ 48. ________ _________________ SrSO3 _______________ 49. ________ _________________ ____________ Silver bromate 50. ________ _________________ ____________ Copper(I) sulfide 51. ________ _________________ ____________ Ammonium sulfate 52. ________ _________________ ____________ Aluminum arsenite 53. ________ _________________ _____________ Iron (II) carbonate 54. ________ _________________ _____________ Sodium dichromate 55. ________ _________________ _____________ Cadmium phosphate 56. ________ _________________ _____________ Cesium bromide 57. ________ _________________ _____________ 58. ________ _________________ __________Potassium permanganate 59. ________ _________________ __________ Iron (III) chloride 60. _______ ________________ ___________ Barium selenate Zinc chlorite C. Other Salts 1. Acid Salts – Salts from which all the replaceable hydrogen ions of the acid have not been removed. NaH2PO4 = Sodium dihydrogen phosphate Na2HPO4 = Sodium hydrogen phosphate NaHCO3 = Sodium hydrogen carbonate (sodium bicarbonate) 2. Basic Salts- Salts from which all the replaceable hydroxyl radicals of the base have not been removed. Al(OH)Br2 Al(OH)2Br = = Hydroxoaluminum dibromide Dihydroxoaluminum bromide 3. Double Salts - Salts containing two different metallic elements or acid radicals. KAl(SO4)2 = MgNH4PO4 = Potassium aluminum sulfate Magnesium ammonium phosphate Name the following Salts: 1. KHSO4 ___________________________________ 2. CaHAsO4 ___________________________________ 3. NaHSO3 ___________________________________ 4. LiHSO3 ___________________________________ 5. NaHCO3 ___________________________________ 6. Na2HAsO3 ___________________________________ 7. CdHPO3 ___________________________________ 8. KHCO3 ___________________________________ 9. NaHSeO4 ___________________________________ 10. Al(HSO4)3 ___________________________________ 11. NH4H2PO4 ___________________________________