8.4-Polarity
advertisement

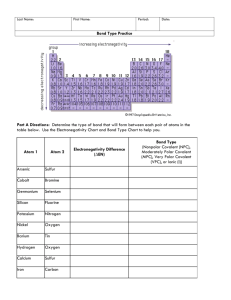
Polar Bonds and Molecules Chapter 8.4 Learning Objectives • Be able to use electronegativity to identify polar vs. non-polar covalent bond • Draw correct dipoles on a covalent bond • Draw correct net dipole on a molecular compound If the difference in electronegativity is between – – – 1.7 to 4.0: Ionic Bond 0.3 to 1.7: Polar Covalent Bond 0.0 to 0.3: Non-Polar Covalent Bond Electronegativity Scale Polar Covalent Bond vs NonPolar Covalent Bond Polar Covalent vs Non-Polar Covalent Drawing Bond Dipoles: BF3 Drawing Bond Dipoles: CH3OH Net Dipole of Molecules Dipole – separation of charges within a molecule between two covalently bonded atoms. Net Dipole – When all the dipole vectors are summed to give one overall dipole. Let’s Practice Get out your white boards … Show the polar bonds (d- and d+) and then predict the net dipole moment of the molecule. You’ll need to use the electronegativity chart on page 177 of your textbook. PF3 - phosphorus trifluoride NH3 - ammonia (nitrogen trihydride) CCl4 – carbon tetrachloride BrF5 - bromine pentafluoride