File
advertisement

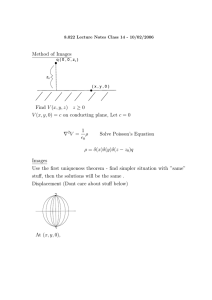
Chapter 10 WS SI Leader: Kristen Kelly Summer 2013 CHM 112 1. Why don't some molecules with polar covalent bonds have dipole moments? 2. Identify the type of intermolecular forces present in the following substances: a. HCl b. CH3CH3 c. CH3NH2 d. Kr e. CHCl3 f. CnH2n +2 g. O2 h. CH3OH i. 3. HF For the following molecule identify if the bond is non-polar covalent, polar covalent, or ionic. Draw out dipole forces in order to assess the polarity. === 4. Methanol (CH3OH) boils nearly 230 °C higher than methane (CH4 B.P. -164 °C) but 1-decanol (C10H21OH, bp = 231 °C) boils only 57 °C higher than decane (C10H22 bp= 172 °C). 4. Why is the dipole moment for SO2 1.63 D but the dipole moment for CO2 is zero? Draw lewis structures to help you explain.