Worksheet-Polarity of Bonds
advertisement

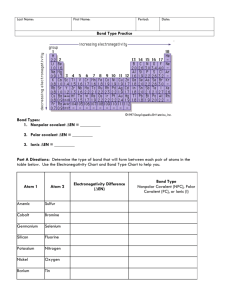
Worksheet-Polarity of Bonds I. Determine the type of bond (ionic, polar covalent, or non-polar covalent ) that will form between atoms of the following elements and show the polarity of the bond if it is polar covalent. a. Ca and Cl b. C and S c. Mg and F d. N and O e. H and O f. S and O II. The bonds between the following pairs of elements are covalent. Arrange them according to polarity, naming the most polar bond first. a. H—Cl b. H—C c. H—F d. H—O e. H—H f. S—Cl III. Using the table of electronegativities from your Periodic table, calculate the EN difference for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons. In your drawing, show which atom is partially positive or partially negative if it is a polar covalent bond. Molecule PCl3 NH3 H2O EN Difference Type of Bond Atom with greater EN Lewis Structure CaO H2S HCl Br2 Na2S H2O2 HCN NI3 SO3 O2 (SO4)2- (PO4)3-