Type 2 DM
advertisement

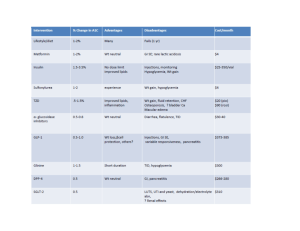
Diabetes mellitus Introduction • It is a clinical syndrome characterized by disturbance in carbohydrates, fat, & proteins metabolism due to either insulin deficiency or insulin resistance. • Clinical presentation: – Polyuria, polydipsia, polyphagia, and weight changes. – Complications of diabetes: recurrent infections, neuropathy, retinopathy, nephropathy, DKA. • Types of diabetes – Type 1 DM: Autoimmune destruction of pancreatic beta cells. There is absolute insulin deficiency – Type 2 DM: Pancreas produces some insulin but either the amount is not sufficient, or there is insulin resistance Preparation of insulins Preparation Onset of effect Peak Activity duration (hours) Rapid-acting insulins ☛ Insulin Lispro ☛ Insulin Aspart Short-acting insulins ☛ Regular insulin 10 – 20 minutes 30 – 60 minutes 3–5 30 – 60 minutes 1 – 2 hours 5–7 1 – 2 hours 6 – 12 hours 18 – 24 4 – 6 hours 1 – 2 hours 16 – 18 hours No peak 24 – 36 24 Intermediate-acting ☛ NPH (Neutral protamine Hagedorn) ☛ Insulin Lente Long-acting insulins ☛ Insulin Ultralente ☛ Insulin glargine Note: All are administered subcutaneously; Regular insulin can be administered I.V., especially in the management of diabetic ketoacidosis; surgery and during acute infections. Mechanism of Action of Insulin • Insulin binds to a specific transmembrane tyrosinekinase linked receptor located in cell membranes of most tissues. The receptor consists of two alpha subunits linked to two beta subunits. • Insulin binding to the alpha subunits causes the activation of the beta receptor subunit, which contain the tyrosine kinase. The enzyme is phosphorylated and this turn leads to the following two cascade pathways: 1. IRS-1 Pathway 2. IRS-2 Pathway Mechanism of Action of Insulin 1. Insulin receptor substrate-1 (IRS-1) pathway: Leading to a. Regulation of proliferation and differentiation of several cell types b. Regulation of DNA synthesis 2. Insulin receptor substrate-2 (IRS-2) pathway: Leading to a. Increased glucose uptake by the lipid and muscle cells b. Increased glycogen formation c. Regulation of gene transcription Pharmcodynamics of Insulin The general physiological function of insulin is to conserve fuel by facilitating the uptake, utilization and storage of glucose, amino acids and fats after meals. Effects on carbohydrate metabolism • Increased glucose transport into the cells • Increased glycogen synthesis • Increased glycolysis • Decreased glycogenolysis • Decreased gluconeogenesis Effects on protein metabolism • Increased amino acid transport into the cells. • Increased protein synthesis. Pharmcodynamics of Insulin Effects on lipid metabolism • Increased triglyceride formation and storage • Decreased lipolysis • Increased lipogenesis (glucose is converted to fat) Other metabolic effects • Increased transport into cells of K+, Ca++, nucleosides and phosphate. Long-term actions • Stimulation of cell proliferation Methods of administration: – S.C. injection. – Portable pen injector. – Pump. Follow up of insulin therapy: – By estimation of glucose in urine using urine dipsticks – From capillary blood glucose level using portable glucometers. Indications of insulin 1. Type 1 DM. 2. Type 2 DM in some conditions: – After failure of oral drugs. – If the patient of type 2 got “stress conditions” e.g. infections, surgery, or pregnancy. 3. Diabetic ketoacidosis: regular insulin is the only type used I.V. 4. Hyperkalemia (insulin + glucose): insulin ↑ shift of K+ from blood to tissues. Side effects of insulin 1. Hypoglycemia: the most common and dangerous side effect. If unconscious treated with IV glucose 2. Hypokalemia: insulin causes shift of K+ from extrato intracellular fluid. 3. Hypersensitivity reactions; with animal insulin 4. Insulin resistance. 5. Local side effects – Lipodystrophy: (atrophy or hypertrophy) of s.c. tissue after repeated injections. – Local infection (rare now). Interaction of Insulin with Other Drugs Drug Interaction Clinical Relevance Alcohol Hypoglycemia (ethanol inhibits gluconeogenesis) High Beta-blockers Prolonged hypoglycemia and masking of certain symptoms of hypoglycemia High Salicylates Hypoglycemia, with large doses (mechanism unknown) Medium Fenfluramine Hypoglycemia (the drug increases the uptake of glucose into striated muscle) Medium MAO inhibitors Hypoglycemia (MAO inhibit gluconeogenesis) Medium Events Requiring an Increase in Dosage of Insulin in Diabetic Patients ✐ Infections ✐ High fever ✐ Trauma, surgical operations ✐ Myocardial infarction ✐ Pregnancy ✐ Hyperthyroidism ✐ Diabetic ketoacidosis Oral antidiabetic drugs 1. Sulfonylureas 2. Meglitinides. 3. Biguanides 4. Thiazolidinediones 5. α- glucosidase inhibitors 1. Sulfonylureas • Classification: 1. First-generation compounds: chlorpropamide, tolbutamide 2. Second-generation compounds: glibenclamide, glyclazide and glipizide. 3. Third-generation compounds: glimepiride Pharmacokinetics • Given 30 minutes before breakfast. • Plasma protein binding is high 90 – 99 %. • Metabolized by liver and their metabolites are excreted in urine with about 20 % excreted unchanged. Mechanism of action: 1. Pancreatic action: • ↑ insulin secretion by pancreatic β cells. • ↓ serum glucagon. 2. Extrapancreatic action: • ↑ insulin receptor sensitivity, • ↓ hepatic output of glucose. Therapeutic uses 1. Type 2 DM (they are not effective in type 1 DM). 2. Chloropropamide may be used in treatment of nephrogenic diabetes insipidus (it ↑ sensitivity of renal tubules to ADH), thus it can cause SIADH Contraindications and Precautions • Type I diabetes (as sole therapy) • Pregnancy (risk of hypoglycemia in the newborn). • Severe liver or kidney disease. • Sulfa drug hypersensitivity. Adverse effects: 1. Hypoglycemia . 2. Increased appetite and weight gain. 3. Pharmacologic failure is common due to progressive decline in β cell function. 4. Hepatotoxicity . 5. Teratogenicity . 6. Allergic reactions. 7. Disulfiram-like reaction in patients ingesting alcohol (chlorpropamide) 8. Dilutional hyponatremia, SIADH (mainly with chlorpropamide) 2. Meglitinides • Repaglinide & nateglinide • They increase insulin secretion . • Fast onset and short duration so; they are taken orally just before meals to control postprandial hyperglycemia. • Hypoglycemia is the major side effect . • They should be used with caution in patients with hepatic or renal impairment . 3. Biguanides • Metformin Mechanism of action: 1. ↑ insulin receptors sensitivity. 2. ↓ intestinal glucose absorption 3. ↓ hepatic gluconeogenesis. • It does not increase insulin secretion (so it doesn’t cause hypoglycemia). Pharmacokinetics • Metformin is well absorbed from small intestine. • Does not bind to plasma proteins • Excreted unchanged in urine. • Half life is 1.5 - 4.5 hours, taken in three doses with meals. Therapeutic uses: 1. Type 2 DM either alone (in mild cases) or in combination with other drugs. 2. To enhance weight loss in obese patients. 3. Polycystic ovary syndrome; it lowers serum androgens and restores normal menstrual cycles and ovulation. Adverse effects: • GIT upset (the most common): anorexia, vomiting, and diarrhea. • ↓ absorption of vitamin B12 • Lactic acidosis with renal failure Contraindications 1. Patients with renal impairment. 2. Patients with hepatic impairment. 3. Past history of lactic acidosis. 4. Chronic lung disease. 4. Thiazolidinediones • Pioglitazone , Rosiglitazone (Insulin sensitizers) Mechanism of action • They bind to a nuclear receptor (peroxisome proliferator activated receptor, PPAR), located mainly in adipose tissue, skeletal muscle and liver, which regulates the transcription of several insulin responsive genes leading the following effects: – ↑ Insulin receptor sensitivity (by about 60%). – ↑ number of glucose transporters →↑ glucose uptake. • Have beneficial effect on serum lipoprotein levels (↓TGs). Therapeutic uses: • To improve insulin resistance in type 2 DM. Adverse effects: • Does not cause hypoglycemia • Hepatotoxicity • Fluid retention leading to peripheral edema & weight gain. (avoid in patients with CHF). 5. αAlpha-Glucosidase inhibitors • Miglitol and acarbose • They act by competitive inhibition of intestinal α-glucosidase enzyme involved in the breakdown of starches into simple sugars → ↓ digestion & absorption of carbohydrates. • Does not cause hypoglycemia • GIT side effects are common: flatulence, diarrhea, abdominal pain. • Elimination: miglitol > 90% by the kidney (avoid in renal failure) • They should be avoided in patients with inflammatory bowel disease. Drug interactions with oral hypoglycemic drugs 1. Drugs potentiate the hypoglycemic effect: – Microsomal enzyme inhibitors. – β-blockers ( it masks the symptoms of hypoglycemia) – Salicylates. 2. Drugs antagonize the hypoglycemic effect: – Microsomal enzyme inducers. – β-agonists. – Thiazides and diazoxide. – Anti-insulin hormones: e.g. steroids and glucagon.