Chapter 18 - UCF Chemistry
advertisement

Chapter 18
Electrochemistry
GOALS
Balancing redox reactions
Voltaic cells
Electrochemical potentials
Electrolysis
…& the calculations!!
Review:
oxidation states
oxidation/reduction
oxidizing/reducing agent
2
ch. 17
Why Study Electrochemistry?
• Batteries
• Corrosion
• Industrial production
of chemicals such as
Cl2, NaOH, F2 and Al
• Biological redox
reactions
The heme group
Electron Transfer Reactions
• Electron transfer reactions are oxidation-reduction
or redox reactions (i.e. changes in oxidation states).
• Redox reactions can result in the generation of an
electric current (battery), or, may be caused by
applying an electric current (electroplating).
• Therefore, this field of chemistry is often called
ELECTROCHEMISTRY.
ELECTRON TRANSFER REACTIONS
0
0
1+
1-
2Na(s) + Cl2(g) 2NaCl(s)
1+
0
2+
0
2HCl(aq) + Zn(s) ZnCl2(aq) + H2(g)
0
0
Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s)
0
0
3+
2-
2Fe(s) + xH2O(l) + 1½O2(g) Fe2O3.xH2O(s)
4+ 2-
0
0
CO2(g) + H2O(l) + energy (CH2O)n + O2(g)
Review of Terminology for Redox Reactions
• OXIDATION—loss of electron(s) by a species;
increase in oxidation number ; e- to the right of arrow.
Na Na+ + e-
• REDUCTION—gain of electron(s); decrease in
oxidation number; e- to left of arrow.
½Cl2(g) + e- Cl-
• OXIDIZING AGENT—electron acceptor; it is
reduced: ½ Cl2(g) + e- Cl-
• REDUCING AGENT—electron donor; it is oxidized
Na Na+ + e-
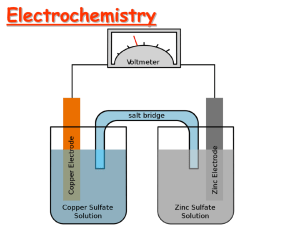
Electrochemical Cells
• Apparatus for generating an
electric current through the use
of a product favored reaction
(spontaneous): voltaic or
galvanic cell.
• An electrolytic cell is used to
carry out electrolysis (an
electric current is used to bring
about a nonspontaneous
chemical reaction).
Batteries are voltaic
cells
Electrochemistry
Alessandro Volta,
1745-1827, Italian
scientist and inventor.
Luigi Galvani, 1737-1798,
Italian scientist and inventor.
Balancing Equations for Redox Reactions
Some redox reactions have equations that
must be balanced by special techniques.
MnO4-(aq) + 5 Fe2+(aq) + 8 H+(aq)
Fe = +2
Mn = +7
Mn2+ (aq) + 5 Fe3+(aq) + 4 H2O(liq)
Mn = +2
Fe = +3
Rules for Assigning Oxidation States
• rules are in order of priority
1. free elements have an oxidation state = 0
Na = 0 and Cl2 = 0 in 2 Na(s) + Cl2(g)
2. monatomic ions have an oxidation state equal
to their charge
Na = +1 and Cl = -1 in NaCl
3. (a) the sum of the oxidation states of all the
atoms in a compound is 0
Na = +1 and Cl = -1 in NaCl, (+1) + (-1) = 0
10
Rules for Assigning Oxidation States
3. (b) the sum of the oxidation states of all the atoms in
a polyatomic ion equals the charge on the ion
N = +5 and O = -2 in NO3–, (+5) + 3(-2) = -1
4. (a) Group I metals have an oxidation state of +1 in all
their compounds
Na = +1 in NaCl
(b) Group II metals have an oxidation state of +2 in
all their compounds
Mg = +2 in MgCl2
11
Rules for Assigning Oxidation States
5. in their compounds, nonmetals have oxidation
states according to the table below (grp # - 8)
nonmetals higher on the table take priority
Nonmetal
Oxidation State
Example
F
-1
CF4
H
+1
CH4
O
-2
CO2
Group 7A
-1
CCl4
Group 6A
-2
CS2
Group 5A
-3
NH3
12
Balancing Equations
Cu + Ag+
give
Cu2+ + Ag
Balancing Equations
Step 1:
Divide the reaction into halfreactions, one for oxidation and the
other for reduction.
Ox
Cu Cu2+
Red
Ag+ Ag
Step 2:
Balance each for mass. Already
done in this case.
Step 3:
Balance each half-reaction for
charge by adding electrons.
Ox
Cu Cu2+ + 2eRed
Ag+ + e- Ag
Balancing Equations
Step 4:
Multiply each half-reaction by a factor
so that the reducing agent supplies as many
electrons as the oxidizing agent requires.
Reducing agent
Cu Cu2+ + 2eOxidizing agent
2 Ag+ + 2 e- 2 Ag
Step 5:
Add half-reactions to give the overall
equation.
Cu + 2 Ag+ Cu2+ + 2Ag
The equation is now balanced for both
charge and mass (the 2e- of the left are
cancelled out with those on the right).
Reduction of VO2 with Zn
+
Balancing Equations
Balance the following in acid solution—
VO2+ + Zn VO2+ + Zn2+
Step 1:
Write the half-reactions
Ox
Zn Zn2+
Red
VO2+ VO2+
Step 2:
Balance each half-reaction for
mass.
Ox
Zn Zn2+ + 2eRed
VO2+ + e- VO2+ excess of 2+ on the right
Zn lost two electrons. They are written on the right.
V is 5+ on the left and 4+ on the right. It gained one
electron. That is written on the left side.
Balancing Equations
Step 3:
Balance half-reactions for charge
Reaction is acidic, then we can use H+.
Ox
Zn Zn2+ + 2eRed
e- + 2 H+ + VO2+ VO2+ + H2O
Step 4:
Multiply by an appropriate factor.
Ox
Zn Zn2+ + 2eRed
2e- + 4 H+ + 2 VO2+
2 VO2+ + 2 H2O
Step 5:
Add balanced half-reactions.
Zn + 4 H+ + 2 VO2+ Zn2+ + 2 VO2+ + 2 H2O
Tips on Balancing Equations
• Never add O2, O atoms, or O2to balance oxygen.
Balance O with OH- or H2O.
• Never add H2 or H atoms to
balance hydrogen.
Balance H with H+/H2O in
acid or OH-/H2O in base.
Tips on Balancing Equations
{Equations that include oxoanions like
SO42-, NO3-, ClO- , CrO42-, and MnO4-,
also fall into this category}.
Be sure to write the correct charges on all the
ions.
•Check your work at the end to make sure mass
and charge are balanced.
•PRACTICE!!!!!!!!!!!
More Practice - Balance the equations below!
I(aq) + MnO4(aq) I2(aq) + MnO2(s) in basic solution
An alkaline (basic) solution of hypochlorite ions reacts with
solid chromium(III) hydroxide to produce chromate and
chloride ions.
ClO3- + Cl- Cl2 (in acid)
Cr2O72- + I- IO3- + Cr3+ (in acid)
MnO4- + H2SO3 SO42- + Mn2+ (in acid)
Cr(OH)4- + H2O2 CrO42- + H2O (in basic soln)
Zn + NO3- Zn(OH)4- + NH3 (in basic soln)
21
VOLTAIC CELLS
- use a chemical rxn to
produce an electric current.
The Zn|Zn2+ and Cu|Cu2+ Cell
Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s)
A CHEMICAL CHANGE PRODUCES AN ELECTRIC
CURRENT
With time, Cu plates out onto Zn metal
strip, and Zn strip “disappears.”
Electrons are transferred from Zn to
Cu2+, but there is no useful electric
current.
Oxidation: Zn(s) Zn2+(aq) + 2eReduction: Cu2+(aq) + 2e- Cu(s)
-------------------------------------------------------Cu2+(aq) + Zn(s) Zn2+(aq) + Cu(s)
A CHEMICAL CHANGE PRODUCES AN ELECTRIC
CURRENT
•To obtain a useful current,
Zn(s) Zn2+(aq) + 2e-
we separate the oxidizing
and reducing agents so that
electron transfer occurs
through an external wire.
This is accomplished in a GALVANIC or VOLTAIC
cell.
A group of such cells is called a battery.
Zn Zn2+ + 2e-
Cu2+ + 2e- Cu
Oxidation
Anode
Negative
Anions
Cations
Reduction
Cathode
Positive
•Electrons travel through external wire.
•Salt bridge allows anions and cations to move
between electrode compartments.
CELL POTENTIAL, E
1.10 V
Zn and Zn2+,
anode
Cu and Cu2+,
cathode
1.0 M
1.0 M
• Electrons are “driven” from anode to cathode
•
by an electromotive force or emf.
For Zn/Cu cell, this is indicated by a voltage of
1.10 V at 25 ˚C and when [Zn2+] = [Cu2+] = 1.0 M.
Need Calculate Cell Voltage
• Balanced half-reactions can be added together
to get the overall, balanced equation.
Zn(s) Zn2+(aq) + 2eCu2+(aq) + 2e- Cu(s)
-------------------------------------------Cu2+(aq) + Zn(s) Zn2+(aq) + Cu(s)
If we know Eo for each half-reaction, we add
them to get Eo for overall reaction.
-need Eo for Zn & Cu half-cells
Standard Reduction Potential
• We can measure ALL other half-cell
potentials relative to another half-reaction.
• We select as a standard half-reaction, the
reduction of H+ to H2 under standard
conditions (1atm, 1 M @ 25 oC) and which
we assign a potential difference = 0 V
Standard Hydrogen Electrode, SHE
28
Zn/Zn2+ half-cell hooked up to a SHE.
Eo for the cell = +0.76 V
Negative
electrode
Positive
electrode
Supplier
of
electrons
Zn Zn2+ + 2eOxidation
Anode
Acceptor
of
electrons
2 H+ + 2e- H2
Reduction
Cathode
Zn is a better reducing agent than H2
Cu/Cu2+ half-cell hooked up to a SHE.
Eo for the cell = +0.34 V
Positive
Negative
Acceptor
of
electrons
Cu2+ + 2e- Cu
Reduction
Cathode
H2 2 H+ + 2eOxidation
Anode
Supplier
of
electrons
H2 is now a better reducing agent than Cu!
Zn/Cu Electrochemical Cell
+
Anode;
negative;
source of
electrons
Cathode;
positive;
sink for
electrons
oxid: Zn(s) Zn2+(aq) + 2eEo = +0.76 V
red: Cu2+(aq) + 2e- Cu(s)
Eo = +0.34 V
--------------------------------------------------------------Cu2+(aq) + Zn(s) Zn2+(aq) + Cu(s)
Eo (calc’d) = +1.10 V
Uses of Eo Values
Organize half-reactions by
relative ability to act as
oxidizing/reducing agents.
Half-rxns are written as
reduction rxns!!
Cu2+(aq) + 2e- Cu(s)
Zn2+(aq) + 2e- Zn(s)
Eo = +0.34 V
Eo = –0.76 V
When a reaction is reversed, the sign of E˚ is reversed!
oxidizing agents
reducing agents
34
Using Standard Potentials, Eo
Which is the best oxidizing agent:
O2 (1.23 V); H2O2 (1.77 V) or Cl2 (1.36 V)?
H2O2 (1.77 V)
Which is the best reducing agent:
Hg (+0.79 V), Al (-1.66 V), or Sn (-0.14 V)?
Al (-1.66 V)
Using Standard Potentials, Eo
Which substance is the best oxidizing agent?
Cr2O72- + 6e- + 14H+ 2Cr3+ + 7H2O
(+1.33 V)
O2 + 4e- + 4H+ 2H2O
(+1.23 V)
Fe3+ + e- Fe2+
(+0.77 V)
Cr2O72Which element/ion is the best reducing agent?
Fe3+ + e- Fe2+
(+0.77 V)
I2 + 2e- 2I(+0.54 V)
Sn4+ + 2e- Sn2+
(+0.15 V)
Sn2+
Standard Redox Potentials, Eo
Any substance on the right
will reduce any substance
HIGHER than it on the
LEFT.
• Zn can reduce H+ and Cu2+.
• H2 can reduce Cu2+ but not
Zn2+
• Cu cannot reduce H+ or
Zn2+.
Standard Redox Potentials, Eo
Ox. agent Cu2+ + 2e- --> Cu
+0.34
2 H+ + 2e- --> H2
0.00
Zn2+ + 2e- --> Zn
-0.76 Red. agent
Any substance on the right will reduce any substance
higher than it on the left.
Northwest-southeast rule: product-favored
reactions occur between
• reducing agent at southeast corner (ANODE) &
• oxidizing agent at northwest corner (CATHODE)
Standard Redox Potentials, Eo
Ox. agent Cu2+ + 2e- --> Cu
+0.34 V
Ni 2++ 2e- --> Ni
-0.25 V
Zn2+ + 2e- --> Zn
-0.76 V
Red. agent
Zn will reduce Ni2+, Cu2+; Ni will reduce Cu2+.
Northwest-southeast rule: product-favored
reactions occur between
• reducing agent at southeast corner (ANODE) &
• oxidizing agent at northwest corner (CATHODE)
Using Standard Potentials, Eo
• In which direction do the following reactions go?
• Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) +0.46 V
• Cu2+(aq) + Zn(s) Cu(s) + Zn2+(aq) +1.10 V
Go to the right as written
• Fe2+(aq) + Cd(s) Fe(s) + Cd2+(aq)
-0.04 V
Goes LEFT, opposite to direction written
• What is Eonet for this reverse reaction?
+0.04 V
Eo for a Voltaic Cell
Fe(s) + Cd2+(aq) Cd(s) + Fe2+(aq)
Cd Cd2+ + 2eor
Cd2+ + 2e- Cd (-0.40 V)
Fe Fe2+ + 2eor
Fe2+ + 2e- Fe (-0.44 V)
Which way does the reaction proceed?
In which direction is it spontaneous?
Eo for a Voltaic Cell
From the table,
• Fe is a better reducing agent
than Cd (-0.44 V; anode)
• Cd2+ is a better oxidizing
agent than Fe2+ (-0.40 V;
cathode)
Eo = E˚cathode - E˚anode (reverse the smaller, more
negative, then add)
cathode: Cd2+(aq) + 2e- Cd(s) -0.40 V (red)
- anode: Fe(s) Fe2+(aq) + 2e- +0.44 V (oxid)
Overall :
Fe(s) + Cd2+(aq) Cd(s) + Fe2+(aq) +0.04 V
More 0n Cell Voltage
When two half-rxns (written as reduction) are
joined in an electrochemical cell, the one with
the larger half-cell potential occurs in the
forward direction, and the one with the smaller
potential occurs in the reverse direction.
Cd2+ + 2e- Cd (-0.40 V)
Fe2+ + 2e- Fe (-0.44 V)
larger
smaller
(reverse this rxn)
Cd2+ + 2e- Cd (-0.40 V)
Fe Fe2+ + 2e- (+0.44 V)
larger
overall: Cd2+ + Fe Cd + Fe2+ (+0.04 V)
More 0n Cell Voltage
When two half-rxns (written as reduction) are
joined in an electrochemical cell, the one with
the larger half-cell potential occurs in the
forward direction, and the one with the smaller
potential occurs in the reverse direction.
Ni2+ + 2e- Ni (-0.23 V)
Mn2+ + 2e- Mn
(-1.18 V)
larger
smaller
(reverse this rxn)
Ni2+ + 2e- Ni
Mn Mn2+ + 2e-
(-0.23 V)
(+1.18 V)
overall: Ni2+ + Mn Ni + Mn2+ (+0.95 V)
More 0n Cell Voltage
Assume I- ion can reduce water.
2 H2O + 2e- H2 + 2 OHCathode
2 I- I2 + 2eAnode
------------------------------------------------2 I- + 2 H2O I2 + 2 OH- + H2
Assuming reaction occurs as written,
E˚net = E˚cathode - E˚anode (from values in table)
= (-0.828 V) - (+0.535 V) = -1.363 V
Minus Enet˚ means net rxn. occurs in the
opposite direction (favors I- + H2O).
I2 + 2 OH- + H2 2I- + H2O (+1.363 V)!!!
Calculate Ecell for the reaction at 25C
Al(s) + NO3−(aq) + 4 H+(aq) Al3+(aq) + NO(g) + 2 H2O(l)
(This is the reaction of Al with nitric acid)
Separate the
reaction into
the oxidation
and reduction
half-reactions
Find the E for
each halfreaction and
sum to get
Ecell
ox:
Al(s) Al3+(aq) + 3 e− Eox = −Ered
= +1.66 V
red: NO3−(aq) + 4 H+(aq) + 3 e− NO(g) + 2 H2O(l)
E°red = +0.96 V
Eox = −(Ered) = +1.66 V
Ered = +0.96 V
Ecell = (+1.66 V) + (+0.96 V) = +2.62 V
46
For the reaction
Al(s) + NO3−(aq) + 4 H+(aq) Al3+(aq) + NO(g) + 2 H2O(l)
ox:
Al(s) Al3+(aq) + 3 e−
Eox = −Ered = +1.66 V
red: NO3−(aq) + 4 H+(aq) + 3 e− NO(g) + 2 H2O(l)
Ered = +0.96 V
Ecell = (+1.66 V) + (+0.96 V) = +2.62 V
The symbol of the cell is
Al(s)| Al3+(aq) || NO3−(aq), 4 H+(aq) , NO(g) |Pt
This is the symbol for the salt bridge
This is the symbol for the electrode-solxn contact
47
E at Nonstandard Conditions
0.0257 V [products]
EE
ln
n
[reactants ]
o
RT
EE
ln Q
• The NERNST EQUATION
nF
• E = potential under nonstandard conditions
o
•
•
•
•
•
n = no. of electrons exchanged
ln = “natural log”
If [P] and [R] = 1 mol/L, then E = E˚
If [R] > [P], then E is LARGER than E˚
If [R] < [P], then E is smaller than E˚
Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s)
Zn is anode (-0.76 V) in 0.40 M Zn2+(aq) and Cu
is cathode (0.34 V) in 4.8 10-3 M Cu2+(aq) .
Calculate the cell potential.
Solution: (need standard cell potential, Eocell)
Eºcell = Eºcathode– Eºanode = (0.34 V) – (–0.76 V)
= 0.34 + 0.76 = +1.10 V
Substituting
E Ecell
2
o
0.0257 V [Zn ]
ln
2
n
[Cu ]
Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s)
Zn is anode (-0.76 V) and Cu is cathode (0.34 V)
n = 2 (Cu2+ + 2e- = Cu0)
Eocell = 1.10 V
Solution:
0.0257 V
[0.40]
E 1.10 V
ln
3
2
[4.8 10 ]
E = 1.10 V – (0.01285)(4.42) = 1.04 V
2Fe3+(aq) + 3Mg(s) 2Fe(s) + 3Mg2+(aq)
Fe3+ = 1.0 10-3 M ; Mg2+ = 2.5 M
Calculate the cell potential.
Solution: (need standard cell potential, Eocell)
Eºcell = Eºcathode– Eºanode = (-0.036 V) – (–2.37 V)
= -0.036 + 2.37 = +2.33 V
Substituting
E Ecell
What is n???
2 3
o
0.0257 V [ Mg ]
ln
3 2
n
[ Fe ]
2Fe3+(aq) + 3Mg(s) 2Fe(s) + 3Mg2+(aq)
Fe3+ = 1.0 10-3 M ; Mg2+ = 2.5 M
Calculate the cell potential.
Solution: (need standard cell potential, Eocell)
Eºcell = +2.33 V; n = 6
Substituting
3
0.0257 V
[2.5]
E 2.33 V
ln
3 2
6
[1.0 10 ]
0.0257 V
E 2.33 V
16.564
6
E 2.33 V 0.071 V
Ans = 2.26 V
BATTERIES
Primary, Secondary, and Fuel Cells
Dry Cell Battery
Primary battery — uses redox
reactions that cannot be
restored by recharge.
Anode (-)
Zn Zn2+ + 2eCathode (+)
2 NH4+ + 2e-
2 NH3 + H2
Alkaline Battery
Nearly same reactions as in common dry
cell, but under basic conditions.
Anode (-): Zn + 2 OH- ZnO + H2O + 2eCathode (+): 2 MnO2 + H2O + 2e-
Mn2O3 + 2 OH-
Lead Storage Battery
• Secondary battery
• Uses redox reactions
•
that can be reversed.
Can be restored by
recharging
Lead Storage Battery
Anode (-) Eo = +0.36 V
Pb + HSO4- PbSO4 + H+ + 2eCathode (+) Eo = +1.68 V
PbO2 + HSO4- + 3 H+ + 2e PbSO4 + 2 H2O
Ni-Cad Battery
Anode (-)
Cd + 2 OH- Cd(OH)2 + 2eCathode (+)
NiO(OH) + H2O + e- Ni(OH)2 + OH-
Fuel Cells: H2 as a Fuel
•Fuel cell - reactants are
supplied continuously from
an external source.
•Cars can use electricity
generated by H2/O2 fuel
cells.
•H2 carried in tanks or
generated from
hydrocarbons.
Storing H2 as a Fuel
One way to store H2 is to adsorb the gas onto a
metal or metal alloy.
Hydrogen—Air (O2) Fuel Cell
Anode: 2H2(g) 4H+(aq) + 4e-
Cathode: O2(g) + 2H2O(liq) + 4e- 4OH- (aq)
---------------------------------Net: O2(g) + 2H2(g) 2H2O(liq)
Electrolysis
Using electrical energy to produce
chemical change.
Sn2+(aq) + 2 Cl-(aq) Sn(s) + Cl2(g)
Electrolysis of water; electroplating; refining metals;
production of chemicals.
Electrolysis
Electric Energy Chemical Change
• Electrolysis of
molten NaCl.
• Here a battery
“pumps” electrons
from Cl- to Na+.
electrons
BATTERY
+
Anode
Cathode
• NOTE: Polarity of
electrodes is
reversed from
batteries.
Cl-
Na+
Electrolysis of Molten NaCl
electrons
Anode (+)
BATTERY
2Cl-(l) Cl2(g) + 2e-
+
Anode
Cathode
Cl-
Na+
(-1.36 V)
Cathode (-)
Na+(l) + e- Na
(-2.71 V)
Eo for cell (in melted NaCl) = E˚c + E˚a
= - 2.71 V + (-1.36 V)
= - 4.07 V (in melted NaCl) rxn is nonspontaneous
External electrical energy needed because Eo is (-).
Electrolysis of Aqueous NaOH
NaOH + H2O Na+(aq) + OH-(aq)
Electric Energy Chemical Change
Anode
Anode (+)
Cathode
E° = -0.40 V
4 OH-
O2(g) + 2 H2O + 4e-
Cathode (-)
4 H2O + 4e-
2 H2 + 4 OHEo for cell = -1.23 V
H2O is more easily reduced
than Na+!! (Eo -2.71 V)
Electrolysis of Aqueous NaCl
NaCl + H2O Na+(aq) + Cl-(aq)
Anode (+)
2 Cl-
Cl2(g) + 2e- E° =-1.36 V
Cathode (-)
2 H2O + 2e-
H2 + 2 OHEo for cell = -2.19 V
Note that H2O is more
Easily reduced than Na+(E° = -2.71 V)
2H2O(l)
Also, Cl- is oxidized in preference to H2O
O2(g) + 4H+ + 4e- because of kinetics (overvoltage)
E°red < -1.23 V, may be down to ~ -2.00 V
Eo and Thermodynamics
• Eo is related to ∆Go, the free energy
change for the reaction.
• ∆G˚ proportional to –nE˚
∆Go = -nFEo
where F = Faraday constant
= 9.6485 x 104 J/V•mol of e(or 9.6485 104 coulombs/mol)
and n is the number of moles of electrons
transferred.
Electrolysis of Aqueous CuCl2
CuCl2 + H2O Cu2+(aq) + 2Cl-(aq)
Anode (+)
2 Cl- Cl2(g) + 2eCathode (-)
Cu2+ + 2e- Cu
Eo for cell = -1.02 V
Note that Cu is more easily
reduced than either H2O or
Na+ (check redox potentials).
Calculate Go for the reaction,
Zn2+(aq) + Ni(s) Zn(s) + Ni2+(aq)
Solution: use Go = -nFE°
no. of electrons, n = 2
F = 9.6485 104 C
need Eocell
Zn2+(aq) + 2e- Zn(s), -0.763 V cathode
Ni(s) Ni2+(aq) + 2e-, +0.25 V anode
Eocell = Ecathode – (Eanode)
Eocell = -0.763 – (-0.25 V) = -0.51 V
Go = (-2 96485 J/V -0.51 V)
1 kJ/1000 J
98 kJ
∆Go > 0, reaction is nonspontaneous
Reagents are favored
Eo and ∆Go
∆Go = - n F Eo
For a product-favored reaction
Reactants Products
∆Go < 0 and so Eo > 0
Eo is positive
For a reactant-favored reaction
Reactants Products
∆Go > 0 and so Eo < 0
Eo is negative
E°cell, G° and K!!
• for a spontaneous reaction
G° < 0 (negative)
E° > 0 (positive)
K > 1 (large)
EE
0
cell
RT
ln Q
nF
When Ecell = 0 (no net rxn), reactants and products are
at equilibrium……and Q = K
o
0
.
0257
nE
cell
0
Ecell
ln K
ln K
72
n
0.0257
Quantitative Aspects of Electrochemistry
Consider electrolysis of aqueous silver ion.
Ag+ (aq) + e- Ag(s)
1 mol e 1 mol Ag
If we could measure the moles of e-, we could
know the quantity of Ag formed.
But do we measure moles of e-?
charge passing
Current =
time
coulombs
I (amps) =
seconds
Quantitative Aspects of Electrochemistry
coulombs
charge passing
I (amps) =
Current =
seconds
time
But , how is charge related to moles of electrons?
=
=
96,500 C/mol e1 Faraday
96,500 C
1 mol e
or
1 mol e
96,500 C
Michael Faraday
1791-1867
Quantitative Aspects of Electrochemistry
I (amps) =
coulombs
seconds
1.50 amps flow thru a Ag+(aq) solution for 15.0 min.
What mass of Ag metal is deposited?
Solution
(a) Calc. charge
Charge (C) = current (A) x time (t)
= (1.5 amps)(15.0 min)(60 s/min) = 1350 C
Quantitative Aspects of Electrochemistry
coulombs
I (amps) =
seconds
1.50 amps flow thru a Ag+(aq) solution for 15.0 min. What mass of Ag metal is
deposited? Ag+ + e- Ag(s)
Solution
(a) Charge = 1350 C
(b) Calculate moles of e- used
1 mol e 1350 C •
0.0140 mol e 96,500 C
(c)
Calc. quantity of Ag
1 mol Ag
0.0140 mol e - •
0.0140 mol Ag or 1.51 g Ag
1 mol e -
Quantitative Aspects of Electrochemistry
The anode reaction in a lead storage battery is
Pb(s) + HSO4-(aq) PbSO4(s) + H+(aq) + 2eIf a battery delivers 1.50 amp, and there is 454 g of Pb, how long will
the battery last?
Solution
a) 454 g Pb = 2.19 mol Pb
b) Calculate moles of eeach Pb atom is loosing 2e−
2 mol e 2.19 mol Pb •
= 4.38 mol e 1 mol Pb
c)
Calculate charge
4.38 mol e- • 96,500 C/mol e- = 423,000 C
Quantitative Aspects of Electrochemistry
The anode reaction in a lead storage battery is
Pb(s) + HSO4-(aq) PbSO4(s) + H+(aq) + 2eIf a battery delivers 1.50 amp, and you have 454 g of Pb, how long will the
battery last?
Solution
a) 454 g Pb = 2.19 mol Pb
b) Mol of e- = 4.38 mol
c) Charge = 423,000 C
d)
Calculate time
Charge (C)
Time (s) =
I (amps)
423,000 C
Time (s) =
= 282,000 s About 78 hours
1.50 amp