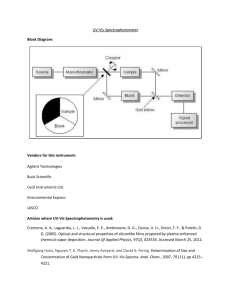
Spectrophotometry Fundamentals: Beer's Law & Absorption
advertisement

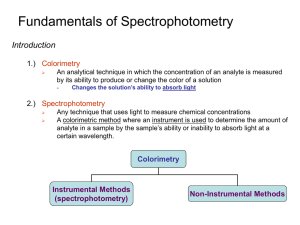
Fundamentals of Spectrophotometry Introduction 1.) Colorimetry An analytical technique in which the concentration of an analyte is measured by its ability to produce or change the color of a solution - Changes the solution’s ability to absorb light 2.) Spectrophotometry Any technique that uses light to measure chemical concentrations A colorimetric method where an instrument is used to determine the amount of analyte in a sample by the sample’s ability or inability to absorb light at a certain wavelength. Colorimetry Instrumental Methods (spectrophotometry) Non-Instrumental Methods Fundamentals of Spectrophotometry Introduction 3.) Illustration Measurement of Ozone (O3) Above South Pole - O3 provides protection from ultraviolet radiation Seasonal depletion due to chlorofluorocarbons O3 cycle Chain Reaction Depletion of O3 Spectra analysis of [O3] Fundamentals of Spectrophotometry Properties of Light 1.) Particles and Waves Light waves consist of perpendicular, oscillating electric and magnetic fields Parameters used to describe light - amplitude (A): height of wave’s electric vector - Wavelength (l): distance (nm, cm, m) from peak to peak - Frequency (n): number of complete oscillations that the waves makes each second Hertz (Hz): unit of frequency, second-1 (s-1) 1 megahertz (MHz) = 106s-1 = 106Hz Fundamentals of Spectrophotometry Properties of Light 1.) Particles and Waves Parameters used to describe light - Energy (E): the energy of one particle of light (photon) is proportional to its frequency E hn where: E = photon energy (Joules) n = frequency (sec-1) h = Planck’s constant (6.626x10-34J-s) As frequency (n) increases, energy (E) of light increases Fundamentals of Spectrophotometry Properties of Light 1.) Particles and Waves Relationship between Frequency and Wavelength ln c n c / l where: c = speed of light (3.0x108 m/s in vacuum)) n = frequency (sec-1) l = wavelength (m) Relationship between Energy and Wavelength E where: hc l ~ hcn n~ = (1/l) = wavenumber As wavelength (l) decreases, energy (E) of light increases Fundamentals of Spectrophotometry Properties of Light 2.) Types of Light – The Electromagnetic Spectrum Note again, energy (E) of light increase as frequency (n) increases or wavelength (l) decreases Fundamentals of Spectrophotometry Properties of Light 2.) Types of Light – The Electromagnetic Spectrum Fundamentals of Spectrophotometry Absorption of Light 1.) Colors of Visible Light Many Types of Chemicals Absorb Various Forms of Light The Color of Light Absorbed and Observed passing through the Compound are Complimentary Fundamentals of Spectrophotometry Absorption of Light 2.) Ground and Excited State When a chemical absorbs light, it goes from a low energy state (ground state) to a higher energy state (excited state) Energy required of photon to give this transition: DE E1 - Eo Only photons with energies exactly equal to the energy difference between the two electron states will be absorbed Since different chemicals have different electron shells which are filled, they will each absorb their own particular type of light - Different electron ground states and excited states Fundamentals of Spectrophotometry Absorption of Light 3.) Beer’s Law The relative amount of a certain wavelength of light absorbed (A) that passes through a sample is dependent on: distance the light must pass through the sample (cell path length - b) amount of absorbing chemicals in the sample (analyte concentration – c) ability of the sample to absorb light (molar absorptivity - e) Increasing [Fe2+] Absorbance is directly proportional to concentration of Fe+2 Fundamentals of Spectrophotometry Absorption of Light 3.) Beer’s Law The relative amount of light making it through the sample (P/Po) is known as the transmittance (T) P T Po Percent transmittance P %T 100 Po T has a range of 0 to 1, %T has a range of 0 to 100% Fundamentals of Spectrophotometry Absorption of Light 3.) Beer’s Law Absorbance (A) is the relative amount of light absorbed by the sample and is related to transmittance (T) - Absorbance is sometimes called optical density (OD) P A - log - log (T ) - log (%T / 100 ) Po A has a range of 0 to infinity Fundamentals of Spectrophotometry Absorption of Light 3.) Beer’s Law Absorbance is useful since it is directly related to the analyte concentration, cell pathlength and molar absorptivity. This relationship is known as Beer’s Law A ebc where: Beer’s Law allows compounds to be quantified by their ability to absorb light, Relates directly to concentration (c) A = absorbance (no units) e = molar absorptivity (L/mole-cm) b = cell pathlength (cm) c = concentration of analyte (mol/L) Fundamentals of Spectrophotometry Absorption of Light 4.) Absorption Spectrum Different chemicals have different energy levels - different ground vs. excited electron states - will have different abilities to absorb light at any given wavelength Absorption Spectrum – plot of absorbance (or e) vs. wavelength for a compound The greater the absorbance of a compound at a given wavelength (high e), the easier it will be to detect at low concentrations Fundamentals of Spectrophotometry Absorption of Light 4.) Absorption Spectrum By choosing different wavelengths of light (lA vs. lB) different compounds can be measured lA lB Fundamentals of Spectrophotometry Chemical Analysis 1.) Calibration To measure the absorbance of a sample, it is necessary to measure Po and P ratio - Po – the amount of light passing through the system with no sample present P – the intensity of light when the sample is present Po is measured with a blank cuvet - Cuvet contains all components in the sample solution except the analyte of interest P is measured by placing the sample in the cuvet. To accurately measure an unknown concentration, obtain a calibration curve using a range of known concentrations for the analyte Fundamentals of Spectrophotometry Chemical Analysis 2.) Limitations in Beer’s Law Results in non-linear calibration curve At high concentrations, solute molecules influence one another because of their proximity - Molar absorptivity changes Affect on equilibrium, (HA and A- have difference absorption) Analyte properties change in different solvents Errors in reproducible positioning of cuvet - Also problems with dirt & fingerprints Instrument electrical noise Keep A in range of 0.1 – 1.5 absorbance units (80 -3%T) Fundamentals of Spectrophotometry Chemical Analysis 3.) Precautions in Quantitative Absorbance Measurements Choice of Wavelength - Choose a wavelength at an absorption maximum - Minimizes deviations from Beer’s law, which assumes e is constant Pick peak in absorption spectrum where analyte is only compound absorbing light Or choose a wavelength where the analyte has the largest difference in its absorbance relative to other sample components Bad choice for either Best choice compound (b) (a) compound (a) or (b) Fundamentals of Spectrophotometry Chemical Analysis 4.) Example: A 3.96x10-4 M solution of compound A exhibited an absorbance of 0.624 at 238 nm in a 1.000 cm cuvet. A blank had an absorbance of 0.029. The absorbance of an unknown solution of compound A was 0.375. Find the concentration of A in the unknown. Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 1.) Molecule Promoted to a More Energetic Excited State Absorption of UV-vis light results in an electron promoted to a higher energy molecular orbital s s* transition in vacuum UV n s* saturated compounds with non-bonding electrons n p*, p p* requires unsaturated functional groups (eq. double bonds) most commonly used, energy good range for UV/Vis Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 1.) Molecule Promoted to a More Energetic Excited State Two Possible Transitions in Excited State - Single state – electron spins opposed Triplet state – electron spins are parallel In general, triplet state has lower energy than singlet state Singlet to Triplet transition has a very low probability Singlet to Singlet Transition are more probable Life-times: 10-5 to 10-8 s 10-4 to 10 s Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 2.) Infrared and Microwave Radiation Not energetic enough to induce electronic transition Change vibrational, translational and rotational motion of the molecule - The entire molecule and each atom can move along the x, y, z-axis When correct wavelength is absorbed, Oscillations of the atom vibration is increased in amplitude Molecule rotates or moves (translation) faster Vibrational States of Formaldehyde Energy: Electronic >> Vibrational > Rotational symmetric Out-of-plane twisting asymmetric In-plane rocking In-plane scissoring Out-of-plane wagging Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 3.) Combined Electronic, Vibrational and Rotational Transitions Absorption of photon with sufficient energy to excite an electron will also cause vibrational and rotational transitions There are multiple vibrational and rotational energy levels associated with each electronic state - Excited vibrational and rotational states are lower energy than electronic state Therefore, transition between electronic states can occur between different vibrational and rotational states Vibrational and rotational states associated with an electronic state Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 4.) Relaxation Processes from Excited State There are multiple possible relaxation pathways Vibrational, Rotational relaxation occurs through collision with solvent or other molecules - energy is converted to heat (radiationless transition) Electronic relaxation occurs through the release of a photon (light) Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 4.) Relaxation Processes from Excited State Internal conversion – transition between singlet electronic states through overlapping vibrational states Intersystem crossing – transition between a singlet electronic state to a triplet electronic state by overlapping vibrational states Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 4.) Relaxation Processes from Excited State Fluorescence – emitting a photon by relaxing from an excited singlet electronic states to a ground singlet state S1 So Phosphorescence – emitting a photon by relaxing from an excited triplet electronic states to a ground singlet state T1 So Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 5.) Fluorescence and Phosphorescence Relative rates of relaxation depends on the molecule, the solvent, temperature, pressure, etc. Energy of Phosphorescence is less than the energy of fluorescence Phosphorescence occurs at a longer wavelengths than fluorescence Lifetime of Fluorescence (10-8 to 10-4 s) is very short compared to phosphorescence (10-4 to 102 s) Fluorescence and phosphorescence are relatively rare Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 5.) Fluorescence and Phosphorescence Fluorescence and phosphorescence come at lower energy than absorbance Emission spectrum is roughly mirror image of absorption spectrum Color Change Due to Fluorescence at Higher Wavelength Fundamentals of Spectrophotometry What Happens When a Molecule Absorbs Light? 5.) Fluorescence and Phosphorescence Emission spectrum are of lower energy or higher wavelength because of the efficiency of vibrational relaxation - Absorption to an excited vibrational state will relax quickly to a ground vibrational state before the electronic relaxation Fundamentals of Spectrophotometry Chemical Analysis 1.) Excitation and Emission Spectra Excitation Spectra – measure fluorescence or phosphorescence at a fixed wavelength while varying the excitation wavelength. Emission Spectra – measure fluorescence or phosphorescence over a range of wavelengths using a fixed varying excitation wavelength. Fundamentals of Spectrophotometry Chemical Analysis 2.) Fluorescence and Phosphorescence Intensity At low concentration, emission intensity is proportional to analyte concentration - Related to Beer’s law I kPo c where: k = constant Po = light intensity c = concentration of analyte (mol/L) At high concentrations, deviation from linearity occurs - Emission decreases because absorption increases more rapidly Emission is quenched absorption of excitation or emission energy by analyte molecules in solution Fundamentals of Spectrophotometry Chemical Analysis 3.) Example In formaldehyde, the transition n p*(T1) occurs at 397 nm, and the np*(S1) transition comes at 355 nm. What is the difference in energy (kJ/mol) between the S1 and T1 states?