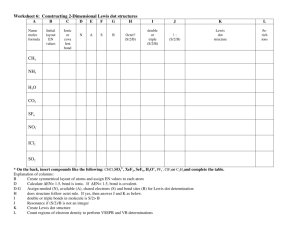
Worksheet 6: Constructing 2-Dimensional Lewis dot structures CH4
advertisement

Worksheet 6: Constructing 2-Dimensional Lewis dot structures A B C Name molec formula Initial layout EN values Ionic or cova lent bond D E F G H I J K L N A S B Octet? = or + | : Lewis dot structure #eregions CH4 NH3 H2O CO2 SF6 NO3ICl2SO2 * On the back, insert compounds like the following: CHCl3SO32-, XeF2, SeF6, H3O+, PF6-, ClF3or C2H2and complete the table. Explanation of columns: B Create symmetrical layout of atoms and assign EN values to each atom D Calculate ΔEN> 1.5, bond is ionic. If ΔEN< 1.5, bond is covalent. D-G Assign needed (N), available (A), shared electrons (S) and bond sites (B) for Lewis dot determination H does structure follow octet rule. If yes, then answer J and K as below. I double or triple bonds in molecule is S/2> B J Resonance if (S/2)/B is not an integer K Create Lewis dot structure L Count regions of electron density to perform VSEPR and VB determinations Constructing 2-Dimensional Lewis dot structures A B C Name molec formula Initial layout EN values Ionic or cova lent bond D E F G H I J K L N A S B Octet? = or + | : Lewis dot structure #eregions