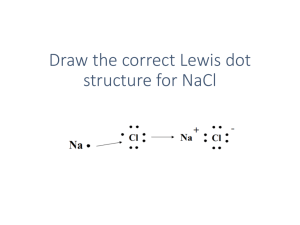
Ionic Bonding and Electron Dot Formulas
advertisement

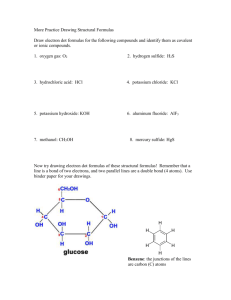
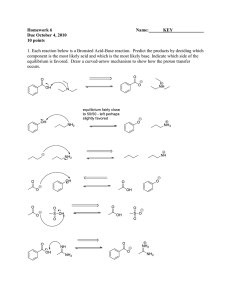
Fun with Ionic Bonding and Lewis Structures Name: _______________ B Period: ____ Date: _____ 1. Write the electron dot formulas for the following atoms: a. Kr e. Sr b. As f. Fr c. H g. I d. He h. Ga 2. Draw the electron dot formulas for the following atoms and then show how the atoms would combine to form neutral compounds (follow the example from your book on page ). Finally, write the chemical formula for each compound. a. Ca and Cl b. K and Br c. Mg and O d. K and S e. Sr and Br f. Ca and P 3. Would xenon and potassium be likely to form an ionic bond? Why or why not? Explain. Fun with Electron-Dot (Lewis) Diagrams Name: _____________ Period: ___ Date:____ Part 1 Use electron dot symbols to represent the formation of the stable ionic compounds from neutral atoms. Use Chapter 11 in your book as a guide. 1. K and I 2. Sr and F 3. Na and S 4. Al and Cl Part 2 Draw electron dot diagrams for the following covalent molecules. 5. Br2 6. NF3 7. CH4 8. HOF 9. H2O2 10. N2H4 11. C2H4 12. HCN B Even More Fun with Lewis Structures B Draw the Lewis structures of the following molecules and draw all resonance structures, if applicable: 1) diphosphorus tetrachloride 2) N2H2O 3) hydrogen peroxide (H2O2) 4) nitrous acid 5) PO3-1 6) dinitrogen monoxide