File - mrs. whalen's classes!
advertisement
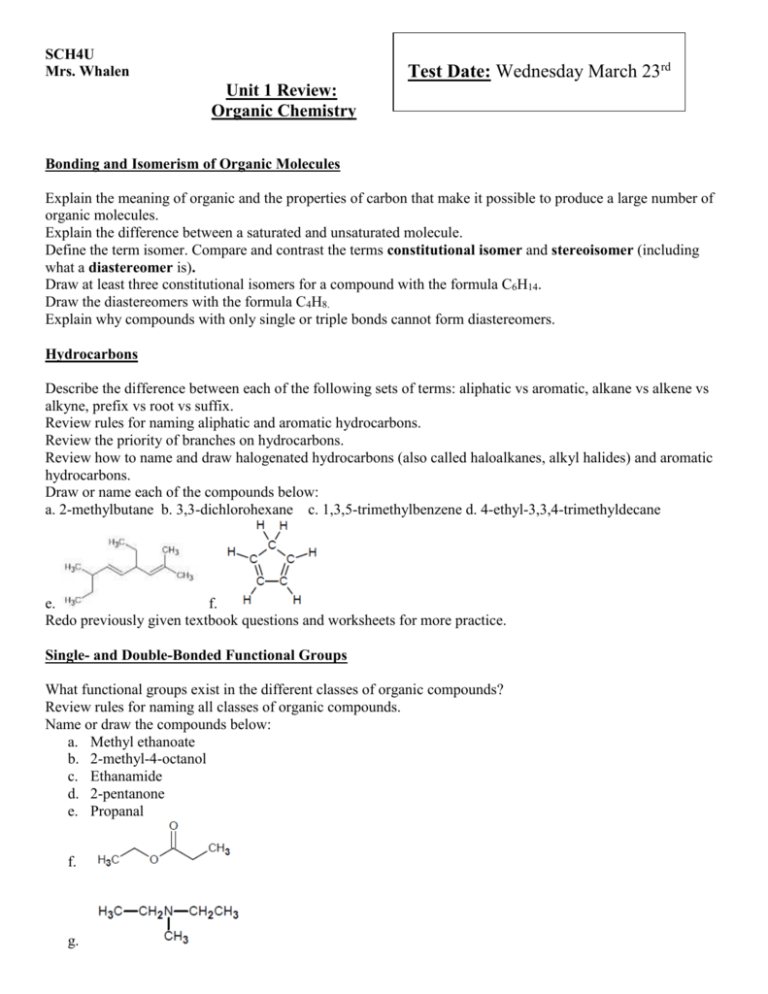
SCH4U Mrs. Whalen Test Date: Wednesday March 23rd Unit 1 Review: Organic Chemistry Bonding and Isomerism of Organic Molecules Explain the meaning of organic and the properties of carbon that make it possible to produce a large number of organic molecules. Explain the difference between a saturated and unsaturated molecule. Define the term isomer. Compare and contrast the terms constitutional isomer and stereoisomer (including what a diastereomer is). Draw at least three constitutional isomers for a compound with the formula C6H14. Draw the diastereomers with the formula C4H8. Explain why compounds with only single or triple bonds cannot form diastereomers. Hydrocarbons Describe the difference between each of the following sets of terms: aliphatic vs aromatic, alkane vs alkene vs alkyne, prefix vs root vs suffix. Review rules for naming aliphatic and aromatic hydrocarbons. Review the priority of branches on hydrocarbons. Review how to name and draw halogenated hydrocarbons (also called haloalkanes, alkyl halides) and aromatic hydrocarbons. Draw or name each of the compounds below: a. 2-methylbutane b. 3,3-dichlorohexane c. 1,3,5-trimethylbenzene d. 4-ethyl-3,3,4-trimethyldecane e. f. Redo previously given textbook questions and worksheets for more practice. Single- and Double-Bonded Functional Groups What functional groups exist in the different classes of organic compounds? Review rules for naming all classes of organic compounds. Name or draw the compounds below: a. Methyl ethanoate b. 2-methyl-4-octanol c. Ethanamide d. 2-pentanone e. Propanal f. g. h. O i. CH3 CH2 CH2 C NH2 O j. H3C C O CH2 CH3 O k. CH3 CH2 CH2 CH Redo previously given textbook questions and worksheets for more practice. Be prepared to compare at least THREE classes of organic compounds in terms of their physical properties. The Main Types of Organic Reactions Define each of the following types of reactions, write general equations where necessary and provide one example of each: addition, substitution, elimination, oxidation, reduction, condensation, hydrolysis. Define Markovnikov’s Rule. Use an example to explain your definition. Redo previously given textbook questions and worksheets for more practice. Textbook Review Questions Chapter 1 Review: p. 87 #1-3, 6, 8-18, 20, 25, 33, 40, 44, 48, 49a-b p. 92 #4, 7-9, 13, 17, 18 Chapter 2 Review: p. 137 #2-5, 7, 8, 15, 23-25, 29-31, 38, 40 Unit Review: p. 147 #2, 3, 7, 8, 9, 10, 12, 15, 16, 18, 22, 26, 27, 28, 29, 30a-c, 31, 33, 35, 38, 44, 46c-d p. 154 #1, 4, 8, 10, 11, 12, 15, 17, 21, 24