Lecture 5
advertisement
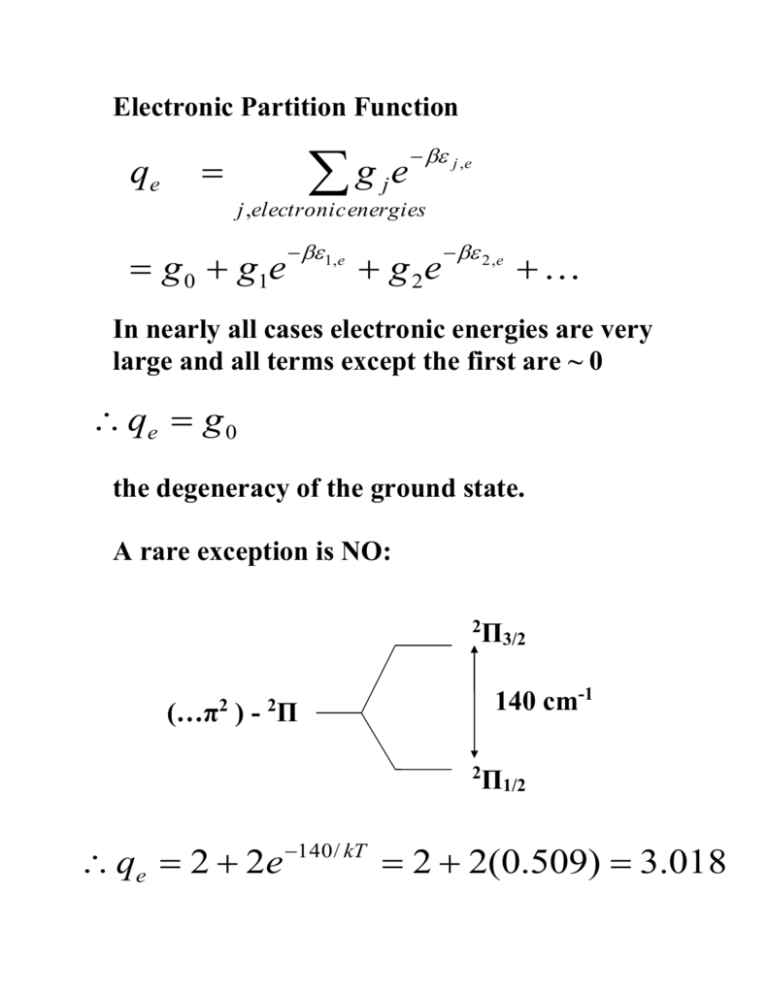
Electronic Partition Function qe g e j ,e j j ,electronic energies g 0 g1e 1,e g 2e 2 ,e In nearly all cases electronic energies are very large and all terms except the first are ~ 0 qe g 0 the degeneracy of the ground state. A rare exception is NO: 2 140 cm-1 (…π ) - Π 2 2 2 qe 2 2e 140 / kT Π3/2 Π1/2 2 2(0.509) 3.018 Overall expression for the molecular partion function of a diatomic molecule: 2 mkT q 2 h 3/ 2 1 kT V h / kT B 1 e and the System partition function: Q q or Q q / N! N N is defined in terms of: m, B, ν, g0 - properties of the molecule N, T, V – properties of the System g0 Magnitude of q. Consider H-35Cl at 300 K m = (36 x 10-3)/NA = 5.978 x 10-26 kg B = 10.54 cm-1 ν ~ 3000 cm-1 g0 = 1 q t = 2.109 x 10 32 V q r = 19.78 qv =1 qe =1 Compare 127 I 2 B = 0.0374 cm-1 ν = 214.5 cm-1 q t = 3.952 x 10 33 V q v = 1.556 q r = 5.575 x 103 qe =1 Units of q t : m (kg), k (J K-1), h (J s) ( kg J K-1 K / J2 s2 )3/2 V (kg J-1 s-2)3/2 V but J – work – force x distance – mass x acceleration x distance – kg m2 s-2 (kg J-1 s-2)3/2 V (kg kg-1 m-2 s2 s-2)3/2 V m-3 V If system contains 1 mole at 1 atmos at 298K, then V ~ 24 l but has to be in m3 ( 1 l = 10-3 m3 ). Justifying the value of β Consider a very simple system and calculate its Internal Energy. System with equally spaced energy levels: E nE , ( n 0,1,2,) Qe e n En n n 1 e e (1 e ) E E but e E nE 2 E e 3 E 1 1 E Q ~ ( E ) 1 which is a very simple result for a very simple system. Now calculate the Internal Energy: 1 Q 1 ( E ) 1 U Q ( E ) 1 1 2 1 ( E ) ( E ) 1 another simple result. U is a state function of the system; (N, T, V ) fixed, U fixed. To change U have to change (N, T, V ) to new, fixed values. But: d U = T dS + p dV and dV = 0 as V is fixed (not variable). Change U of the system by changing T . Therefore: U~T, β -1 ~ T ; β ~ T -1 β = ( kT ) -1 The Boltzmann distribution for individual molecules. Have been using the Boltzmann distribution of Systems in an Ensemble. Ei n e N e i Ei i T to define the average energy per System < E>. Now consider the N (identical) molecules in a System with average energy < ε > E N n j j j N e j N q N j E q q E N n e j j q j N e j n j j N e j j j q j N j q The fraction of molecules in the nth energy level. An application of the Internal Energy expression. The Equipartition Theorem and Heat Capacities. The Equipartition Theorem states: That when quantum effects can be ignored, the average energy of each ‘squared term’ of molecular energy is ½ kT . translational energy: ½ mvx2 + ½ mvy2 +½ mvz2 3/2 kT rotational energy: ½ IA ωA2 + ½ IB ωB2 + ½ IC ωC2 3/2 kT (kT for a linear as only 2 rotational axes) vibrational energy: ½ μ v2 + ½ kx2 kT per vibrational mode This means that at a temperature T there is (on average) as much energy in translation as there is in rotation (can seldom ignore the quantum effects in vibrational motion). 1 Q N q U Q q q qt qr qvt qe N (qt qr qvt qe ) U qt qr qv qe N (qt ) N (qr ) qt qr N N ( qv ) N ( qe ) qv qe t r v e average translational energy per molecule, etc. Now consider each ‘bit’ of the molecular energy.