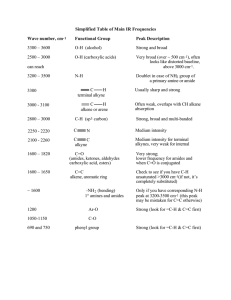
IR Spectroscopy Basic ideas about IR frequencies, interpretation of IR spectra A change in dipole moment during vibration is the essential condition for a molecule to be IR active Homework: Convert 2.5 mM – 15 mM into cm-1 Why do we need IR spectrum • Characterization • Infrared spectrum can be used for molecules much as a fingerprint can be used for humans. • IR spectrum can be used to determine structural information of a molecule Different modes of vibrations Asymmetric stretching frequency is always greater than symmetric stretching frequency Stretching frequencies for C-H and C-D? Take away from last class What is spectroscopy What are electromagnetic radiations What is apparently the most important electromagnetic radiation How do we see objects What is IR spectroscopy and why do we need it Different modes of vibrations How to determine IR frequencies: Comparative study Factors affecting IR frequencies of a molecule. C-H (sp3) = 3100 cm-1 C-H (sp2) = 3200 cm-1 C-H (sp) = 3300 cm-1 C-C = 1220 cm-1 C-N = 1240 cm-1 C-O = 1260 cm-1 = 2150 cm-1 = 2250 cm-1 N-H = 3500 cm-1 O-H = 3600 cm-1 C=C = 1660 cm-1 C=N = 1690 cm-1 C=O = 1720 cm-1 C=C (aromatic) = 1600 cm-1 Comparison between OH, NH and CH Take Away From previous class Important stretching frequency ranges you need to remember CO, C=C, OH, NH, CH characteristic features Hydrocarbons: Alkane, Alkene, Alkynes Trans vs cis OR symmetric vs asymmetric characteristics Alcohol and Phenols Intermolecular and intramolecular hydrogen bonding effect Effect of dilution Ethers Often, two closely spaced C=O absorption peaks are observed for these conjugated systems, resulting from two possible conformations, the s-cis and strans. The s-cis conformation absorbs at a frequency higher than the s-trans conformation. In some cases, the C=O absorption is broadened rather than split into the doublet. Aldehydes Ketones Distinguish between aldehydes and ketones The C-H stretching vibrations found in aldehydes (-CHO) at about 2750 and 2850 cm-1 are extremely important for distinguishing between ketones and aldehydes. If the 2750-cm−1 band is present together with the proper C=O absorption value, an aldehyde functional group is almost certainly indicated. The doublet that is observed in the range 2860–2700 cm−1 for an aldehyde is a result of Fermi resonance. The second band appears when the aldehyde C-H stretching vibration is coupled with the first overtone of the medium-intensity aldehyde C-H bending vibration appearing in the range 1400–1350 cm−1. Tautomers Carboxylic Acid A carboxylic acid exists in monomeric form only in very dilute solution, it absorbs at about 1760 cm−1 because of the electron-withdrawing effect. Acids in concentrated solution, in the form of neat liquid, tend to dimerize via hydrogen bonding. This dimerization weakens the C=O bond and lowers the stretching force constant K, resulting in a lowering of the carbonyl frequency of saturated acids to about 1710 cm−1. The most characteristic feature in the spectrum of a carboxylic acid is the extremely broad O-H absorption occurring in the region from 3400 to 2400 cm-1. This band is attributed to the strong hydrogen bonding present in the dimer In some aromatic acid chlorides, one may observe another rather strong band, often on the lower frequency side of the C=O band, which makes the C=O appear as a doublet. This band, which appears in the spectrum of benzoyl chloride at about 1730 cm-1, is probably a Fermi resonance band. The characteristic pattern for noncyclic and saturated anhydrides is the appearance of two strong bands, not necessarily of equal intensities, in the regions from 1830 to 1800 cm-1 and from 1775 to 1740 cm-1. The two bands result from asymmetric and symmetric stretch. Problems 1. The order of carbonyl stretching frequency in the IR spectra of ketone, amide, and anhydride is: A. ketone > amide > anhydride B. amide > ketone > anhydride C. anhydride > kenone > amide D. anhydride > amide > ketone 2. 3. 4. 5. 6. The frequency of vibration of a bond is a function of which factor? a) Force constant of the bond b) Masses of the atoms involved in bonding c) Force constant of the bond and Masses of the atoms d) Bond order 7. What is the order of decreasing vibrational frequency for C — Cl, C — Br, C — C, C — O and C — H? a) C-H, C-C, C-O, C- Cl, C-Br b) C- Cl, C-Br, C-C, C -H, C-O c) C-O, C-H, C-Br, C- Cl, C-C d) C-Br, C- Cl, C-C, C-O, C-H 8. What is the correct increasing order of stretching frequencies for C ≡ C, C = C and C — C? a) C — C > C = C > C ≡ C b) C ≡ C > C = C > C — C c) C — C > C = C < C ≡ C d) C ≡ C < C — C > C = C 9. Why in the IR spectrum of aromatic acid chloride, a weak band near 1730 cm-1 is formed? a) b) c) d) Inductive effect Fermi resonance Conjugation effect Hyperconjugation effect 10. Why ketenes absorb in IR at a very high frequency (2150 cm-1)? a) The inner C is sp hybridized b) The more s character in a bond, the stronger it is c) Inner C is sp2 hybridized d) Inner C is sp3 hybridized 11. What is the effect of ring strain in lactone (cyclic ester) or a lactam (cyclic amide)? a) Increases carbonyl stretching frequency b) Decreases carbonyl stretching frequency c) Increases C = C frequency d) Decreases C = C frequency 12. What is the relation between wave number of IR absorption and the reduced mass? a) Wave number is directly proportional to reduced mass b) Wave number is inversely proportional to reduced mass c) Wave number is independent of the reduced mass d) Wave number is directly proportional to square of reduced mass 13. Which of the following molecules will not show infrared spectrum? a) H2 b) HCI c) CH4 d) H2O 14. Why Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1? a) Mesomeric (M) effect is dominant in acids over the inductive (I) effect b) I effect is dominant in carboxylic acids over the mesomeric effect c) I effect on ketones is dominant over the M effect d) M effect in ketones is dominant 15. Which is the correct order of increasing wave number of the stretching vibrations of (1) C-H (alkane), (2) O-H (alcohol), (3) C=O (ketone), and (4) C≡C (alkyne)? a) (4) < (3) < (2) < (1) b) (3) < (4) < (2) < (1) c) (3) < (4) < (1) < (2) d) (4) < (3) < (1) < (2) 16. Which of the following statements regarding IR spectroscopy is wrong? a) Infrared radiation is higher in energy than UV radiation. b) Infrared spectra record the transmission of IR radiation. c) Molecular vibrations are due to periodic motions of atoms in molecules, and include bond stretching, torsional changes, and bond angle changes. d) Infrared spectra give information about bonding features and functional groups in molecules. 17. Which is the correct order of increasing wave number of the stretching vibrations of (1) C-H (alkane), (2) C-H (alkene), (3) C-H (alkyne), and (4) C-H (arene)? a) (1) < (2) ≈ (3) < (4) b) (4) < (3) ≈ (2) < (1) c) (3) < (4) ≈ (2) < (1) d) (1) < (4) ≈ (2) < (3) 18. The solution of methyl salicylate in CCl4 shows O-H stretching frequency at 3200 cm- 1. Upon high dilution, the O-H stretching frequency changes to a) 3300 cm- 1 b) 3500 cm- 1 c) 3000 cm- 1 d) Does not change 19. When alcohols or phenols are determined as pure (neat) liquid films, as is common practice, a broad O-H stretching vibration is obtained at 3350 cm−1. As the alcohol is diluted considerably with carbon tetrachloride, a) A sharp peak should appear at about 3000 cm- 1 b) A broad peak should appear at about 3000 cm- 1 c) A sharp peak should appear at about 3600 cm- 1 d) No observable change happens 20. A pair of weak bands, one at 2860–2800 cm−1 and the other at 2760–2700 cm−1 appears in case of a) Ketone b) Aldehyde c) Ester d) Amide 1. (c) 2. (b) 3. (d) 4. (a) 5. (c) 6. (c) 7. (a) 8. (b) 9. (b) 10. (a) 11. (a) 12. (b) 13. (a) 14. (b) 15. (c) 16. (a) 17. (d) 18. (d) 19. (c) 20. (b)