The atomic structure
advertisement

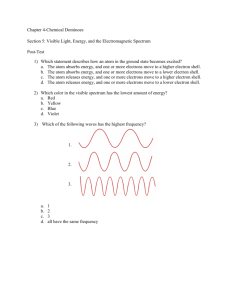
The atom Mass Spectrometer Electron arrangement Electron configuration The atom • State the position of protons, neutrons and electrons in the atom. • State the relative masses and relative charges of protons, neutrons and electrons. • Define the terms mass number (A), atomic number (Z) and isotopes of an element. • Deduce the symbol for an isotope given its mass number and atomic number. • Calculate the number of protons, neutrons and electrons in atoms and ions from the mass number, atomic number and charge. • Compare the properties of the isotopes of an element. • Discuss the uses of radioisotopes State the position of protons, neutrons and electrons in the atom. • Protons and neutrons are present in the nucleus of an atom, electrons are in orbits or shells around the nucleus. • Atomic number, Z = number of protons; the fundamental characteristic of an element. Relative masses and relative charges of protons, neutrons and electrons. • Relative masses: p = 1, n = 1, e = 1/1840; charges: p = +1, n = 0, e- = -1. The atom • Isotopes are atoms of the same element having the same atomic number / proton number but different mass number/ number of neutrons • Isotopes differ in physical properties that depend on mass such as density, rate of diffusion etc. • Chemical properties are the same because of the same electronic configuration or arrangement. The atom • Atomic mass of an atom is the average of the atomic masses of its isotopes; depends on isotopes relative abundance; leads to noninteger atomic masses. • Atomic number is the number of protons in the nucleus of an atom. Radioactive isotopes of all elements can be produced by exposing the natural element to a flux of slow moving neutrons in a nuclear reactor. This results in the nucleus of the atom capturing an additional neutron. Radiocarbon dating •The rate of radioactive decay of carbon-14, can be used to date objects. •Naturally occurring carbon in living organisms contains a fixed proportion of carbon-14 owing to exchange with carbon in the atmosphere. •On death this interchange stops and the proportion of carbon-14 starts to decrease. After about 5,700 years the proportion of carbon-14 will have fallen to about half its initial value. • PEOPLE HAVE CLAIMED THAT THIS SHROUD WAS USED TO RAP THE BODY OF JESUS. • ISOTOPES STUDY GAVE ANOTHER ANSWER Radioisotopes as tracers •Another use of radioisotopes is as “tracers”. this relies on the fact that the radioactive isotopes behave chemically, and thus biologically, in an identical manner to the stable isotopes. •The activity of the thyroid gland, which preferentially absorbs iodine, can be measured by monitoring the increase in radioactivity of the gland after taking a drink containing traces of iodine radioisotopes (typically 125I and 131I). Radioisotopes in radiotherapy •Some radiosotopes produce gamma rays and hence can be a source of quite intense radioactivity. •`Cobalt-60 is an example of this and radiation from cobalt-60 sources is used in radiation treatment for cancer and industrially in devices such as those monitoring the thickness of steel plate from a rolling mill. Mass Spectrometer • Describe and explain the operation of a mass spectrometer. • Describe how the mass spectrometer may be used to determine relative atomic mass using the 12C scale. • Calculate non-integer relative atomic masses and abundance of isotopes from given data. Mass Spectrometer Mass Spectrometer • Stages of Operation: – – – – – Vaporization of sample ionization to produce M+ ions acceleration of ions by electric field deflection of ions by magnetic field detection of ions. Mass Spectrometer • Region A contains the vapourised substance. If it is already a gas, then it will contain the gas at low pressure, if the sample is a solid or liquid, it must be heated to produce the vapour. Mass Spectrometer • In region B, the particles are converted from neutral atoms or molecules into positive ions. This is usually done by bombarding them with fast moving electrons that are accelerated between the two plates shown. These electrons collide with electrons in the particle knocking them out and leaving a positive ion. Mass Spectrometer • In region C, these positive ions are accelerated by the high electrical potential difference between the two parallel electrodes with holes in their centers. Mass Spectrometer • In region D these fast moving ions enter a magnetic field produced by an electromagnet. The poles, shown as circles, are above and below the plane of the diagram. This causes the fast moving ions to deflect Mass Spectrometer • Particles of a certain mass (dependent on the field strength) will continue round the tube and strike the detector plate. Those with a greater mass will not be deflected as much and those with a smaller mass (deflection depends on the charge to mass ratio m/z). Mass Spectrometer • In region E particle will be detected by means of the current flow required to neutralise the positive charge that they carry - the greater the number of particles of a given mass that are present, the greater the current. Mass Spectrometer • Degree of deflection: • Lower the mass, higher the deflection. • Higher the charge, higher the deflection. • Deflection reflects mass/charge ratio; for charge of +1, deflection depends on mass. Mass Spectrometer • For an element, the mass spectrum gives two important pieces of information: the number of isotopes, and the abundance of each isotope; thus the relative average atomic mass, Ar can be calculated. Mass Spectrometer • For a molecule, the highest peak represents the molecular (parent) ion and its mass gives the relative molecular mass, Mr of the compound (and the fragmentation pattern can help determine its structure). Electron Arrangement • Describe the electromagnetic spectrum. • Distinguish between a continuous spectrum and a line spectrum. • Explain how the lines in the emission spectrum of hydrogen are related to electron energy levels. • Deduce the electron arrangement for toms and ions up to Z = 20 Atomic emission spectrum • The study of the emission of light by atoms and ions is the most effective technique for deducing the electronic structure of atoms. • The term “light” is being used rather loosely to indicate electromagnetic radiation. This covers radiation from gamma rays through to radio waves Electromagnetic spectrum • Electromagnetic waves have a range from very low energy waves –like radio waves- and very-high energetic waves like gamma radiations. Emission spectra • Each element gives its characteristic set of colours . • These colours could be observed by a spectrometer as lines each of fixed wavelength called emission spectrum. • Emission spectra are not continuous but consist of separate lines. • These lines become closer ( converge towards the higher energy end of the spectrum Bohr atomic structure • The electron travels in orbits around the nucleus of the atom. • When an electron absorbs energy it moves to a higher level. • When an electron moves down to a lower level it emits a packet of energy called quantum. • Each packet corresponds to a certain wavelength and shows a certain colour. • Continuous spectrum was not observed which meant that electrons can only exist in specific levels but not in between them. • The value of the energy level n=1,2,3….. Is called the principle quantum number Bohr Atomic model • Electrons falling from the outer levels to level 1 will emit the highest amount of energy, and the spectra will be in the UV region. • Electrons falling to level 2 will form a spectrum that falls in the visible region. • Electron falling to level 3 will form a spectrum that falls in the infra red region n=3 n=2 n=1 Hydrogen visible spectrum • The emission spectrum of hydrogen shows four discrete lines in the visible region. • The lines are converging towards the violet which is more energetic. • There are other line spectrum for hydrogen in the invisible region Convergence limit and ionization energy •If sufficient energy is given to the atom, it is possible to excite the electron beyond the highest energy level. •The electron will escape and the atom will become an ion. 1.Why do these levels mean that the atom will show an emission spectrum of discrete lines rather than a continuous spectrum. 2.Which three of the lettered energy changes involve absorption of energy by the atom? 3.Which three levels involves emission? 4.Of the three energy changes that involve emission, one results in blue light, one results in yellow light and the third results in ultraviolet light 1. Which lettered change involve the emission of blue light? 2. Which lettered change involve the emission of yellow light? 3. Which lettered change involve the emission of ultraviolet light? 4 3 A B C D E F 2 1 Emission Spectrum • When electrons are excited, they jump to higher energy levels. • Electrons fall back to lower energy levels, and the energy equivalent to the difference in energy level is emitted in the form of photons. Emission Spectrum • A continuous spectrum contains light of all wavelengths in the visible range. • A line spectrum consists of a few lines of different wavelengths. Emission Spectrum • Energy levels come together in terms of energy the farther away they are from the nucleus; this explains the convergence of lines in a line spectrum. Electron configuration • Explain how evidence from first ionization energies across periods accounts for the existence of main energy levels and sub-levels in atoms. • Explain how successive ionization energy data is related to the electron configuration of an atom. • State the relative energies of s, p, d and f orbitals in a single energy level. • State the maximum number of orbitals in a given energy level. • Draw the shape of an s orbital and the shapes of the px, py and pz orbitals. • Apply the Aufbau principle, Hund’s rule and the Pauli exclusion principle to write electron configurations for atoms and ions up to Z=54 Ionization energy • The ionisation energy of an atom is the minimum amount of energy required to remove a mole of electrons from a mole of gaseous atoms to form a mole of gaseous ions. • This change is endothermic because work is needed to remove the electron. Ionization energy • Factors affecting the magnitude of the ionization energy: –the charge on the nucleus. –Shielding of electrons in Filled inner orbitals. –repulsion that the electron experiences from other electrons within the same shell. Ionization energy Ionization energy • Going down a group of the periodic table, the ionisation energy of the elements decreases. • This is because of a reduction in the amount of electron-electron repulsion and hence the greater nuclear-electron attraction that results causes the remaining electrons to move closer to the nucleus. Ionization Energy Ionization energy • going across a period (for example period 2 from Li to Ne, or period 3 from Na to Ar), it can be seen that the ionisation energy increases. • This is because of the increase in the charge on the nucleus which, as the electrons being removed are all in the same energy level, increases the effective nuclear charge, and hence the ionisation energy. Ionization energy • The more electrons that have been removed from an atom, the greater the energy required to remove the next electron. Electron configuration • The maximum number of electrons in a main energy level n is 2n2: • 1st energy level, n = 1; maximum 2 e-; • 2nd energy level n = 2, maximum 8 e-; • 3rd energy level n = 3, maximum 18 e-. Electron configuration • The electron arrangement (or configuration) indicates the number of electrons and their energy distribution. This determines an element’s physical and chemical properties. Electron configuration • Main energy levels, sub-levels and orbitals: • The main energy levels, n are assigned whole number integers, n = 1, 2, 3, 4… . n = 1 represents the lowest energy level. • Each main energy level contains n sublevels and a total of n2 orbitals. • s, p, d, f etc. is the common notation for sub-levels and orbitals within sub-levels. Electron configuration • An orbital is an area of space around the nucleus in which an electron moves. • Orbitals have characteristic shapes. There is one s orbital which is spherical in shape, three p orbitals which are dumbbell shaped, called px, py pz, and arranged in the x, y, and z directions respectively, five d orbitals and seven f orbitals (both with complex shapes). The relative energies of s, p, d, and f orbitals with in a sub-level are: s < p < d < f. Electron configuration Electron configuration • Each orbital can have a maximum of 2 electrons. –n = 1 has one sub-level which is called an s sub-level and which contains one s orbital. –n = 2 has two sub-levels: 2s and 2p; –n = 3 has 3 sub-levels: 3s, 3p and 3d; –n = 4 has 4 sub-levels:4s, 4p, 4d and 4f, etc. Electron configuration • Each energy sub-level is divided into orbitals each of which can contain up to two electrons, which must have opposite spins. • The Pauli exclusion principle, says that no two electrons in an atom can be in exactly the same state (that is, they cannot be in the same place at the same time). Electron configuration • The electrons in atoms always adopt the lowest energy configuration possible by filling one sub-level completely before starting to fill the sublevel of next highest energy. This is known as the ‘Aufbau’ (building up) principle. Electron configuration • Hund’s rule, states that sub-level orbitals are singly occupied as far as possible by electrons with the same spin. Electron configuration • The orbitals are filled with electrons according to the following order. Electron configuration o Which one of the following represents the 2p orbital of Carbon Electron configuration • The arrangement of electrons in the porbital of some atoms is Electron configuration • The electronic structures of the elements are related to the position of the element in the periodic table.


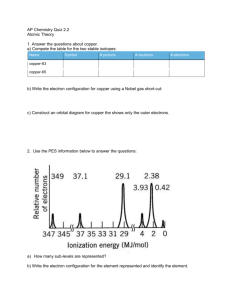

![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)