Atomic Theory Quiz
advertisement
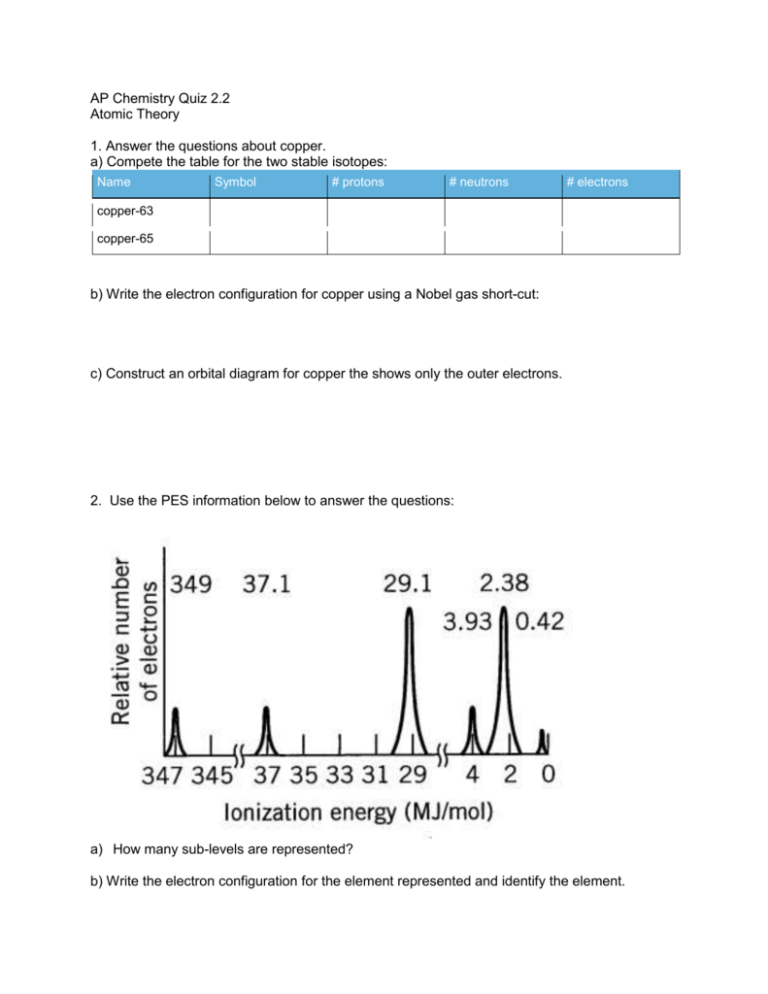
AP Chemistry Quiz 2.2 Atomic Theory 1. Answer the questions about copper. a) Compete the table for the two stable isotopes: Name Symbol # protons # neutrons # electrons copper-63 copper-65 b) Write the electron configuration for copper using a Nobel gas short-cut: c) Construct an orbital diagram for copper the shows only the outer electrons. 2. Use the PES information below to answer the questions: a) How many sub-levels are represented? b) Write the electron configuration for the element represented and identify the element. 4. Sketch the PES spectrum of oxygen on the PES spectrum of sulfur shown below. Select the best answer. 1. ___ How many orbitals make up the 4d sub-shell? a) 0 b) 1 c) 3 d) 5 e) 7 2. ___ The correct electron configuration for nitrogen is 2 2 6 2 2 2 6 4 2 2 3 2 2 2 2 5 a) 1s 2s 2p 3s 3p 2 b) 1s 2s 2p 2d c) 1s 2s 2p 1 d) 1s 2s 3s 4s e) 1s 1p 3. ___ Which of the following elements would have only 1 peak in a photoelectron spectrum? a) b) c) d) e) Ne He Ba Na Li 4. a) b) c) d) e) Which element’s atoms have 8 electrons in “s orbitals”? Na Ca Mg Be He 6. ___ For which element would the attraction of the nucleus for the inter-most electron be the greatest? a) b) c) d) e) H He Be Mg U 7. ___ A PES spectrum shows information to determine: a) electron spin b) c) d) e) the number of neutrons in the nucleus distribution of electrons in sub-levels only those electrons in the outermost energy level the quantum tunneling effects of each electron











