Microanalysis in Science and Engineering
advertisement

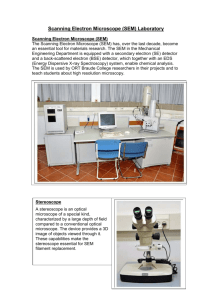
Microanalysis in Science and Engineering - Electron Microscopy A Workshop for Middle and High School Teachers sponsored by Tennessee Technological University Center for Manufacturing Research Departments of Chemical, Mechanical, Earth Sciences and Curriculum and Instruction and The National Science Foundation Faculty Joseph J. Biernacki (Chemical Engineering) June 16, 2003 What will we learn What is electron microscopy? How are electrons generated? How are electrons focused? How do electrons interact with matter? How are the electron/matter interactions used to generate images? What linkages can be made between the “technology fundamentals” and the middle/high school science curriculum? What is electron microscopy? Electron microscopy is an imaging technology that uses the properties of electrons rather than light. A bit of history: e- Source Anode 1st lens 2nd lens Final lens von Ardenne (1938) – earliest recognizable work describing scanning electron microscope (SEM) Zworykin, Hillier and Snyder (1942) – basis for modern SEM Cambridge Scientific Instruments (1965) – “introduction of first commercial instrument” http://mse.iastate.edu/microscopy/path.html http://mse.iastate.edu/microscopy/elementary.html Detectors Backscatter eX-ray Secondary e- I’ve heard other terms used… Electron Probe Microanalyzer (EPMA) An electron probe microanalyzer utilizes X-rays emitted due to electron bombardment to obtain qualitative and quantitative microanalysis. Electron Microprobe (same as EPMA) Transmission Electron Microscope (TEM) Uses transmitted electrons instead of emitted electrons. Scanning Transmission Electron Microscope (STEM) Combines aspects of both SEM and TEM. Environmental Scanning Electron Microscope (ESEM) Similar to a SEM, but does not require the high vacuum. Scanning Auger Microscope (SAM) Similar to an SEM only it uses Auger electron emissions instead of secondary electron emissions for imaging and compositional analysis. http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_Microscopes.html How are electrons generate? Thermionic emission – – Tungsten (W) filament Lanthanum hexaboride (LaB6) filament Field emission The amount of electrons (flux or current density) determines resolution. The size of the electron beam (spot size) determines resolution. http://mse.iastate.edu/microscopy/source.html Suggested Curriculum Links Chemistry and Physics: Work function Thermionic emissions Electrons will escape from heated metals when the thermal energy of the electron is greater than the work function. E Ew Ew=E-EF EF Highest free energy state (Fermi level) Lowest free energy state Recall that the work function is the amount of energy required to remove an electron from its highest free energy state to infinity. Suggested Curriculum Links Physics: current density flux concept Electron flux (current density) current density = AcT2e-Ew/kT Ac=a constant that depends on the material To increase the current density at constant T, either Ac must increase of Ew must decrease. Material W LaB6 Ew (eV) 4.5 2.4 Suggested Curriculum Links Physics: E-field near a sharp object Electron tunneling effect Field emissions V1 V2 Benjamin Franklin discovered that static discharges are attracted to the sharp tip of a conductor. He used this phenomena to invent the lightning rod which he gave as his “gift to the world.” An extremely high field is produced at the sharp tip of the cathode. This reduces the potential barrier and permits electrons to tunnel out. Suggested Curriculum Links Chemistry and Physics: absolute and relative pressure scales The requirement of high vacuum Electrons have extremely low mass (~1/1000 that of a proton) and easily give up their energy in collisions with gas atoms and molecules. SEM technology is not possible without a high vacuum in at least the source and focusing column of the machine. – – – Column vacuum ~10-7 torr Sample chamber vacuum ~<10-5 torr ESEM technology permits sample chamber vacuum ~<20 torr Suggested Curriculum Links Physics: force on a moving charged particle in an B-field Focusing a beam of electrons A magnetic field exerts a force perpendicular to the plane formed by the vector velocity and the magnetic field vector. e- Source FB ev B Anode F 1st lens B v 2nd lens x y Final lens Detectors Backscatter eX-ray z http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_Focus.html http://mse.iastate.edu/microscopy/electro_lens.html http://mse.iastate.edu/microscopy/path2.html Secondary e- Suggested Curriculum Links Chemistry and Physics: kinetic theory, collision dynamics, probability, flux concept How does the e- beam interact with matter? Incident electrons interact with matter in two ways – – elastic collisions inelastic collisions From these interactions, information regarding shape, composition, crystal structure, electronic structure, internal electric or magnetic fields, … Eo N Q , nt ni A N o Q Q=collision cross-section (probability) N=num of collisions/unit volume nt=number of targets/unit volume ni=number of incident particles/unit area (flux) Ei fe http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_Interaction.html http://mse.iastate.edu/microscopy/beaminteractions.html http://biology.udayton.edu/SEM/Principle/2_Imaging.htm Learning about secondary electrons. Use the internet page below and any other webbased resource available to you and what you have learned thus far to answer the following questions about secondary electrons: – – – – – Do secondary electrons originate only from the sample surface? What is the kinetic energy of secondary electrons? What type of interaction produces a secondary electron? What type of information can be obtained from secondary electron emissions? Why is secondary electron emission independent of atomic number? http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_SE1.html Learning about electron interactions Download the software below and use it to answer the following questions: – – – What affect does atomic weight have on the interaction volume? What is the nominal shape of the interaction volume? What affect does beam voltage have on the interaction volume? Design a computational experiment to answer each question. State your design briefly, one or two sentences with a table, etc. Be prepared to present your results in some understandable form. Casino a software for performing Monte Carlo simulation of electron-matter interactions. Inelastic emissions Inelastic interactions result in a wide variety of emissions: – – – – Secondary electrons Characteristic X-rays Bremsstarahlung (continuum) X-rays Cathodluminescence radiation (IR, UV and visible light) Suggested Curriculum Links Physics: electrostatic phenomena How is a secondary image generated? Emitted electrons are not assembled by the electron microscope in the way that light (visible photons) are assembled by the human eye. Light reflecting from a given spot enters the eye. Many points of such reflected light are assembled in a pattern on the eye that exactly mimics the reflecting source. This is not the case for electrons in the electron microscope. incident e beam incident light emitted e- secondary edetector eye ~+12,000 V http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_Basics2.html Suggested Curriculum Links Across the curriculum: computers and information processing How is a secondary image generated? Secondary electrons are generated by the interaction of the incident electron beam and the sample. The secondary electrons emerge at all angles. These electrons gathered by electrostatically attracting them to the detector. Knowing both the intensity of secondary electrons emitted and position of the beam, an image is constructed electronically. incident e- beam beam location emitted e- secondary edetector signal intensity ~+12,000 V http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_Basics1.html http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_se2.html http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_Basics3.html Suggested Curriculum Links Chemistry and Physics: kinetic theory, collision dynamics Elastic collisions Elastic collisions produce backscattered electrons (BS). E i fe Eo Q( fo ) 1.62 10 20 Z=the atomic number E=electron energy (keV) fo=scattering angle Z2 2 fo cot E2 2 In elastic scattering Ei~=Eo. The elastic collision is with the nuclei of an atom, partly obscured by the electron cloud. http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_bse1.html http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_bse2.html http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_bse3.html Detecting BS electrons There are many types of detectors, only the solid state type is discussed here. incident e- beam solid state BS detector BS esample http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_bse4.html What are some unique properties of BS electrons? Deeper penetration Intensity is function of atomic weight of sample (b) Summary SEM used the properties of e- to produce images. e- are generated by a thermionic process wherein the work function of the source must be exceeded. A strong electric field can also be used to permit e- to tunnel out. W is the most common thermal source. Magnets are used to focus the e- beam. The interaction of high energy e- with matter produces either elastic or inelastic collisions. Elastic collisions are responsible for backscattering of e-. Inelastic collisions produce secondary electrons as well as characteristic X-rays and other forms of radiation that give information about the surface morphology, composition, electrical and magnetic properties and crystal structure. Secondary images are not constructed by reflection as with light, but require electrons to be attracted to a detector and assembled using the signal intensity and beam location information. SEM provides many opportunities to connect the science behind the technology with curricular topics in chemistry and physics. Some web links How does and electron microscope work? http://mse.iastate.edu/microscopy/choice.html Electron microscopy basics. http://biology.udayton.edu/SEM/ A more advanced web site about electron microscopy. http://emalwww.engin.umich.edu/emal/courses/SEM_lectureCW/SEM_frontpage.html