Chapter 8
advertisement

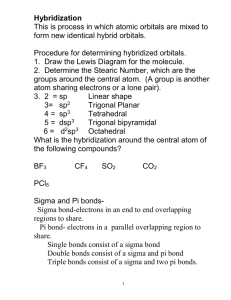
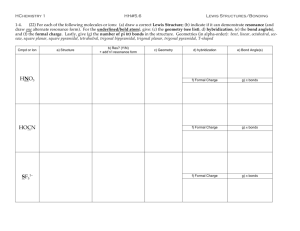
Chapter 8 Molecular Structure, Valence Bond Theory, and Hybridization Valence Shell Electron Pair Repulsion Theory Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral VSEPR Theory • Explains how molecules obtain their shapes. • Coulomb’s Law allows us to predict that regions of High Electron Density (bonds or lone pairs) arrange themselves around a central atom as far away from each other as possible so as to minimize repulsive forces. VSEPR Theory • The combination of Lewis diagrams with the VSEPR Theory gives a powerful model for predicting structural properties of many covalently bonded molecules and polyatomic ions, including: – Molecular Geometry – Bond Angles – Presence of a dipole moment (polarity). From Chapter 8 Note Supplement • Molecular Shape: When explaining molecular shape include the following 3 steps in your answer: – What is the geometry of the molecule: based on the number of regions of high electron density. (See Table 8.1 pp. 215). Discuss VSEPR theory: The regions of high electron density will get as far apart as possible from one another in order to minimize repulsive forces. (The underlined statement is an essential part of any explanation of molecular shape and is the essence of VSEPR theory). What is the shape of the molecule: based on the number of bonds vs. unshared pairs. (See Table 8.2 pp. 216). – – • Explain placement of unshared pairs and/or bonds. (Easiest to use bond angles here). Table 8.1 page 215 Trigonal Planar Table 8.1 Page 215 The Number of Regions of High Electron Density around the Central Atom Determine Molecular Geometry.... 2 regions linear (Bond angle = 180o) 3 regions trigonal planar (Bond angle = 120o) 4 regions tetrahedral (Bond angle = 109.5o) 5 regionso trigonal bipyramidal (Bond angles = 90o and 120 ) 6 regions octahedral (Bond angle = 90o) Trigonal Bipyramidal Axial atoms are above and below the plane of the triangle on opposite sides of the molecule. Equatorial atoms are 120° apart and are within the plane of the triangle. Molecular Shape and VSEPR Theory Helps to Determine 1. Bond Angles 2. Molecular Polarity Table 8.1 page 215 Trigonal Planar Bond Angles • Bond angles are based on the geometry of the molecule • Slight adjustments may be necessary because of various space requirements for different types of regions of high electron density. • Lone pairs have the greatest space requirement followed by triple bonds then double bonds and finally single bonds which require the least amount of space around the central atom. Linear • Two atom molecule. • 2/0 central atom count. – Two atoms attached to the central atom and no lone pairs on the central atom. • Bond angle of 180° Linear Lewis Structure Molecular Geometry O=C=O Linear Lewis Structure CO2 has two regions of high electron density resulting in a linear geometry. In order to minimize the repulsive forces the two oxygen atoms are bonded 180˚apart resulting in a linear shape. Molecular Geometry 3/0 or 2/1 count on central atom. •The geometry of the molecule is trigonal planar. •The base angle for this geometry is 120°. •The shape can be either trigonal planar or angular. Trigonal Planar Trigonal Planar BF3 has three regions of high electron density resulting in a trigonal planar geometry. In order to minimize the repulsive forces the three fluorine atoms are bonded 120° apart resulting in a trigonal planar shape. Explain why NO2- has a bond angle of 115.4°? NO2- has three regions of high electron density which results in a trigonal planar geometry and a base angle of 120°. However the greater space requirement of the lone pair repels the bonds and results in a bond angle of 115.4°. 4/0 or 3/1 or 2/2 count on central atom. •The geometry of the molecule is tetrahedral. •The base angle for this geometry is 109.5°. •The shapes can be either tetrahedral, trigonal pyramidal, or angular. Why does water have a bond angle of 105°? Why does water have a bond angle of 105°? • The four regions of high electron density surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsive forces. This results in a tetrahedral geometry in which the H-O-H bond angle would be 109.5°. However, the two lone pairs around the oxygen atom, have a greater space requirement, effectively pushing the two hydrogen atoms closer together. The result is a H—O—H angle of 105°. 5/0 or 4/1 or 3/2 or 2/3 count on central atom. •The geometry of the molecule is trigonal bipyramidal. •The base angles for this geometry are 120° and 90°. However be careful with the angles on this geometry as they can vary depending upon the shape. •The shapes can be either trigonal bipyramidal, seesaw, T- shaped, or linear. It will be helpful when explaining shapes based on a Trigonal Bipyramidal geometry to remember the difference between axial and equatorial. Axial atoms are above and below the plane of the triangle on opposite sides of the molecule. Equatorial atoms are 120° apart and are within the plane of the triangle. a a a I3 - I3 - I3 - a 6/0 or 5/1 or 4/2 count on central atom. •The geometry of the molecule is octahedral. •The base angles for this geometry 90°. •The shapes can be either octahedral, square pyramidal, or square planar. a a Xenon Tetrafluoride Octahedral Geometry Square Planar Shape Explain the shape of IBr3 • Draw Lewis Structure Explain the shape of IBr3 • • • • • Draw Lewis Structure State VSEPR Theory (MRF) and Geometry Place the Lone Pairs Place the Bonds State the Shape Explain the shape of Xenon Tetrafluoride (XeF4) Molecular Models Skeletal Formulas (Models) Skeletal Formulas (Models) • A skeletal formula shows the atoms that make up a molecule and serves as a shorthand representation of its bonding. • Benefit: They are the most common models of molecules because they can relatively quickly allow us to show the bonding in a molecule. Drawback: They are ineffective at showing the three dimensional structure of the molecule and the relative size of the atoms. Ball-and-Stick Models Ball-and-Stick Models • The ball-and-stick model is used to display both the three-dimensional position of the atoms and the bonds between them. • Benefit: Bond angles and distances between atoms are more clearly shown. • Drawback: In a ball-and-stick model, the pegs used to represent bonds and spheres used to represent atoms are not to scale. As a consequence, the model does not provide a clear insight about the space occupied by the model. Space-Filling Models Space-Filling Models Space-Filling Models • A space-filling model is a three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic nuclei. • Benefit: They are useful for visualizing the effective shape and relative dimensions of the molecule, in particular the region of space occupied by it. • Drawback: Space-filling models do not show the chemical bonds between the atoms, nor the structure of the molecule beyond the outside layer of atoms. Molecular Polarity • A dipole is anything with a positive end and a negative end. Another word for dipole is polar. • A bond is a dipole (polar) if it connects different atoms. • A polar molecule (dipole) is a molecule where the polar bonds are asymmetrically (not symmetrically) arranged (the dipoles do not cancel). • A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged (the dipoles cancel). Dipole Moment • The dipole moment is the measurement of a molecules polarity. • Arrow points toward the more electronegative atom. + H Cl Determining Molecular Polarity • Polar Molecule – Dipoles (Polar Bonds) are asymmetrically arranged and don’t cancel in this bent (angular) molecule. O H2O H H net dipole moment Determining Molecular Polarity • Therefore, polar molecules have... – asymmetrical charge distribution resulting in a positive and negative end to the molecule and a net dipole moment. – the dipoles don’t cancel H CHCl3 Cl Cl Cl net dipole moment Determining Molecular Polarity • Nonpolar Molecules – Dipoles (Polar Bonds) are symmetrically arranged and cancel out in this trigonal planar shaped molecule. F BF3 B F F Hybridization – The Methane Dilemma Carbon 1s22s22p2 Fig. 10.7 Fig. 10.8 Hybridization takes a certain number of different atomic orbitals and mixes (hybridizes) them to create an equal number of equivalent hybrid orbitals. 1. ONLY the CENTRAL ATOM(s) hybridize. 2. The number of regions of high electron density must equal the number of orbitals that hybridize. Atomic orbitals hybridization # hybrid of orbitals # of regions of HED Atomic orbital Even though other types of hybridization exist you will only be responsible for sp, sp2 and sp3 hybridization. hybridization # of regions of HED # hybrid of orbitals What type of hybridization does each central atom exhibit? What type of hybridization does B exhibit? What type of hybridization does Be exhibit? Covalent Bonding • Covalent bonds result when: – atomic orbitals of adjacent atoms overlap. – such overlaps lead to the formation of sigma and pi bonds. Sigma () Bonds • Result from the overlap of: – Two s orbitals – An s orbital and a p orbital – End to end overlap of two p orbitals • Single bonds are sigma bonds. Pi () Bonds • Result from the side to side overlap of two p orbitals. overlap Pi () Bonds • Result from the side to side overlap of two p orbitals. • Double bonds are a sigma bond and a pi bond. • Triple bonds are a sigma bond and two pi bonds. C-C 1 bond C=C 1 bond 1 bond C 1 bond 2 bonds C How many sigma bonds are in this molecule? How many pi bonds? H H C=C H H Hybridization and Type of Bonding (sigma and pi) in Ethylene, CH2CH2 or (C2H4) Determine the hybridization for each central atom. H H C=C H H sp2 hybrids and unhybridized p-orbital bond involve the hybrid orbitals on the central atoms 1 electron from the sp2 hybrid on C, the other from the hydrogen 1s orbital bond results from the side-by-side overlap of the unhybridized p-orbitals Electron from the unhybridized p-orbital on the C atom • • Sigma () Bonding in Ethylene Pi () Bonding in Ethylene Acetylene C2H2 Acetylene C2H2 Covalent Bonding: Sigma and Pi Bonds • The overlap of atomic orbitals is greater in sigma bonds than pi bonds which is illustrated by the greater bond energy of sigma bonds vs. pi bonds. • This is why pi bonds are easier to break than sigma bonds. The pi bond is weaker than the sigma bond, easier to break, making alkenes much more reactive than alkanes. This reaction occurs more readily Covalent Bonding: Sigma and Pi Bonds • The presence of pi bonds prevents the rotation of the bond and leads to different geometric isomers. • Geometric isomers have the same bonding but the atoms spatial arrangements are different. Covalent Bonding: Sigma and Pi Bonds • The presence of pi bonds prevents the rotation of the bond and leads to different geometric isomers. • Geometric isomers have the same bonding but the atoms spatial arrangements are different. Pi Bonding and Delocalization of Electrons • Extended pi bonding can exist in structures such as benzene which allows for the delocalization of pi electrons. • This lead to the discovery in the 1970’s of molecular substances that could conduct electricity. Benzene Benzene