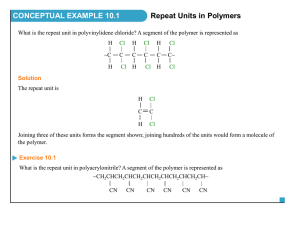
슬라이드 1
advertisement

9 CHAPTER REACTIONS OF VINYL POLYMERS 9.1 Introduction 9.2 Functional 9.3 Ring-forming reactions 9.4 Crosslinking 9.5 Block and graft copolymer formation 9.6 Polymer degradation 9.1 Introduction Applications of chemical modifications : Ion-exchange resins Polymeric reagents and polymer-bound catalysts Polymeric supports for chemical reactions Degradable polymers to address medical, agricultural, or environmental concerns Flame-retardant polymers Surface, treatments to improve such properties as biocompatibility or adhesion, to name a few The purpose of this chapter : To summarize and illustrate chemical modifications of vinyl polymers. 9.1 Introduction Five general categories (1) reactions that involve the introduction or modification of functional groups (2) reactions that introduce cyclic units into the polymer backbone (3) reactions leading to block and graft copolymers (4) crosslinking reactions (5) degradation reactions Polymer reaction시 고려사항 (1) molecular weight (2) crystallinity (3) conformation, steric effect (4) neighboring group effect (5) polymer physical form의 변화 9.1 Introduction neighboring group effect CH3 CH2C CH2CH C O C O O O- O CH3 H2 C CH CH2C C O O - - OH + C O NO2 CH3 CH2C NO2 CO2- CH2CH CO2- (9.1) 9.2 Functional Group Reactions 9.2.1 Introduction of new Functional Groups Chlorination CH2CH2 Cl2 (-HCl) CHCH2 (9.2) Cl Cl2, SO2 (-HCl) Cl2 chlorosulfonation CH2CH2 (-HCl) CH2CH2 CH2CH SO Cl CHCH22 Cl CH2CH2 Cl2, SO2 (-HCl) CH2CH SO2Cl (9.3) 9.2 Functional Group Reactions 9.2.1 Introduction of new Functional Groups Properties of polyethylene by chlorination ① Flammability – decrease ② Solubility – depending on the level of substitution ③ Crystalline – more (under heterogeneous conditions) ④ poly(vinyl chloride) – Tg increase Chlorosulfonation – provides sites for subsequent crosslinking reactions 9.2 Functional Group Reactions 9.2.1 Introduction of new Functional Groups Fluorination CH2CH C6H5 F2 (-HF) CF2CF C6F11 (9.4) Teflon - to improve solvent barrier properties. Aromatic substitution reactions (nitration, sulfonation, chlorosulfonation, etc.) occur readily on polystyrene useful for manufacturing ion-exchange resins useful for introducing sites for crosslinking or grafting 9.2 Functional Group Reactions 9.2.1 Introduction of new Functional Groups introducing new functionalities Chlorometylation CH2CH CH2CH CH3OCH2Cl AlCl3 + CH3OH (9.5) CH2Cl Introduction of ketone groups – via the intermediate oxime NOH CH2CH2 NOCl CCH2 O H2O + H CCH2 (9.6) 9.2.2 Conversion of Functional Groups Reason useful To obtain polymers difficult or impossible to prepare by direct polymerization. alcoholysis of poly(vinyl acetate) CH2CH OCCH3 CH3OH CH2CH OH + CH3CO2CH3 (9.7) O (unstable enol form of acetaldehyde) 9.2.2 Conversion of Functional Groups Examples 1. Saponification of isotactic or syndiotactic poly(trimethylsily methacrylate) to yield isopactic or syndiotactic poly(methacrylic acid) CH3 CH2C C CH3 - (1) H2O, OH CH2C + O (2) H (9.8) CO2H OSi(CH3)3 2. Hofmann degradation of polyacrylamide to give poly(vinyl amine) - CH2CH CONH2 Br2, OH CH2CH NH2 (9.9) 9.2.2 Conversion of Functional Groups 3. Synthesis of “head-to-head poly(vinyl bromide)” by controlled brominaion of 1,4-polybutadiene CH2CH CHCH2 Br2 CH2CH CHCH2 Br Br (9.10) 9.2.2 Conversion of Functional Groups Other types of “classical” functional group conversions dehydrochlorinaion of poly(vinyl chloride) LiCl CH2CH CH CH N,N-dimethylformamide (9.11) Cl hydroformylation of polypentenamer CH CO, H 2 CH(CH2)3 catalyst CH2CH(CH2)3 (9.12) CHO hydroboration of 1,4-polyisoprene CH3 CH3 (1) B2H6 CH2C CHCH2 (2) NaOH, H2O2 CH2CHCHCH2 OH (9.13) 9.2.2 Conversion of Functional Groups Conversion of a fraction of the chloro groups of poly(vinyl chloride) to cyclopentadienyl (CH3)2Al(C5H5) CH2CH CH2CH + (CH3)2AlCl (9.14) Cl Converting the end groups of telechelic polymers. Dehydrochlorination of chlorine-terminated polyisobutylene CH3 CH2CCl - + t-BuO K CH3 CH 3 - + t-BuO K 3 CH2CH CCl Subsequent epoxidation ArCO3H CH2CCH3CH2 CH3 CH2C CH2 ArCO3H CH3 CH2C CH2 (9.15) CH3 32 CH2C CH CH CH2C CH2 O CH3 CH2C CH2 O (9.16) 9.3 Ring-Forming Reactions Introduction of cyclic units greater rigidity higher glass transition temperatures improved thermal stability – carbon fiber (graphite fiber) C C N C N N N N Ladder graphite-type polymer O O O2 (-HCN) (-N2) N N N N N N N H H H H H H SCHEME 9.1. Reactions involved in pyrolysis of polyacrylonitrile to form carbon fiber. highly crosslinked graphitelike polymer 9.3 Ring-Forming Reactions Ladder structures - Poly(methyl vinyl ketone) by intramolecular aldol condensation - OH CH2CH CH2CH CH2CH C O C O C O CH3 CH3 CH3 CH2 (9.17) CH3 O Nonladder structures - Dechlorination of poly(vinyl chloride) CH2CHCH2CH Cl Cl Zn CH2 CH2CH CH (9.18) 9.3 Ring-Forming Reactions Cyclization reaction be made to approach its theoretical limits. CH2CHCH2CH OH RCHO CH2 + H OH O O (9.19) R when R = C3H7, commonly called poly(vinyl butyral) Plastic film Commercially important cyclization - epoxidation of natural rubber CH3 CH3 CH2C CHCH2 H2O2 CH3CO2H CH2C CHCH2 O (9.20) 9.3 Ring-Forming Reactions Rubber and other diene polymers undergo cyclization in the presence of acid cis-1,4-polyisoprene + H + etc. H (9.21) + Quantitative cyclization of 1,2-polybutadiene - with metathesis catalysts Metathesis catalyst + 2CH2 CH2 (9.22) 9.4 Crosskinking 9.4.1 Vulcanization RO + CHCH2 + CHCH2 CH2CH CHCH2 (9.24) CHCH2 CHCH CHCH2 CHCH2 CHCH CHCH2 CHCH CHCH2 CHCH CHCH2 CHCH + CHCH2 CHCH CHCH2 CHCH CHCH2 CHCH CHCH2 CHCH2 CHCH + + RO CH2CH CH2CH CHCH2 (9.23) + ROH CHCH2 CH2CH + ROH (9.25) (9.26) (9.27) CHCH2 CHCH CHCH2 CHCH CHCH2 + (9.28) 9.4 Crosskinking 9.4.1 Vulcanization The oldest method of vulcanization involving addition to a double bond to form an intermediate sulfonium ion CH2CH CHCH2 + + S S CH2CH CHCH2 + S (9.29) S- + then abstracts a hydride ion CH2CH CHCH2 + S CH2CH CH2CH2 + S CH2CH + CHCH2 (9.30) + CH2CH CHCH2 + CH2CH CH CH + CH2CH S Termination - by reaction between sulfenyl anions and carbocations CHCH2 (9.31) 9.4 Crosskinking 9.4.1 Vulcanization Rate of vulcanization increase by the addition of accelerators or organosulfur compounds accelerator S S 2+ (CH3)2NCS-- 2Zn2+ (CH3)2NCS 2Zn 1 zinc salts of dithiocarbamic acids organosulfur compounds S S S S (CH3)2NCSSCN(CH3)2 (CH3)2NCSSCN(CH3)2 2 tetramethylthiuram disulfide 9.4.2 Radiation Crosslinking When vinyl polymers are subjected to radiation crosslinking and degradation priority of reaction Generally, both occur simultaneously Degradation predominates with high doses of radiation With low doses the polymer structure determines which will be the major reaction. Disubstituted polymers tend to undergo chain scission With monomer being a major degradation product 9.4.2 Radiation Crosslinking poly(-methylstyrene), poly(methyl methacrylate), polyisobutylene - decrease in molecular weight on exposure to radiation halogen-substituted polymers ~poly(vinyl chloride) - break down with loss of halogen most other vinyl polymers - crosslinking predominates A limitation of radiation crosslinking that radiation does not penetrate very far into the polymer matrix The method is primarily used with films 9.4.2 Radiation Crosslinking Mechanism of crosslinking Radiation CH2CH2 CH2CH2 (neighboring chain) + H CH2CH2 CH2CH2 + H2 CHCH2 Radiation + H (9.32) + H2 CHCH2 + H2 CHCH2 CHCH2 + H2 (9.33) (neighboring chain) fragmentation reactions CH2CH R CHCH R R + H CH CH + RH (9.34) 9.4.3 Photochemical Crosslinking (Photocrosslinking) Applications electronic equipment printing inks coatings for optical fibers varnishes for paper and carton board finishes for vinyl flooring, wood, paper, and metal curing of dental materials Two basic methods (1) incorporating photosensitizers into the polymer, which absorb light energy and thereby induce formation of free radicals (2) incorporating groups that undergo either photocycloaddition reactions or light-initiated polymerization 9.4.3 Photochemical Crosslinking (Photocrosslinking) (1) incorporating photosensitizers into the polymer, which absorb light energy and thereby induce formation of free radicals When triplet sensitizers (benzophenone) are added to polymer O (들뜬상태) n O UV흡수 radical 생성 Benzophenone CH2CHCH2CH CH2CHCH2CH C O C O R R C O hv O (9.35) -cleavage of the excited + RC R CH2CH2 + CH2 C C O C R R O (9.36) chain cleavage 9.4.3 Photochemical Crosslinking (Photocrosslinking) Poly(vinyl ester) : -cleavage reaction CH2CH C O hv CH2CH OR (9.37) O + C OR (2) incorporating groups that undergo either photocycloaddition reactions or light-initiated polymerization 2 + 2 cycloaddition cyclobutane crosslinks 9.4.3 Photochemical Crosslinking O (a) O OC CH CHAr OC + ArCH Ar hv CH CO Ar O CO O (b) + hv SCHEME 9.2. Photocrosslinking (a) by 2 + 2 cycloaddition and (b) by 4 + 4 cycloaddition. TABLE 9.1. Group Used to Effect Photocrosslinking21-58 Type Structure Alkyne R C C R Anthracene continued Benzothiophene dioxide S O O O Chalcone ArCH CHCAr Cinnamate ArCH CHCO2R R Coumarin R O continued TABLE 9.1. (continued) Type Structure R N Dibenz[b, f]azepine Diphenylcyclopropenecarboxylate CO2S Episulfide H 2C CH2 O R N Maleimide (R=H, CH3, Cl) R' R O continued TABLE 9.1. Type Structure + Stilbazole R N CH CHR Y- Stilbene ArCH CHAr CH CH2 Styrene R N 1,2,3-Thiadiazole R N S O CH3 NH Thymine O N H 9.4.3 Photochemical Crosslinking Photo – reactive groups 의 도입 방법 ① 중합 반응 동안에 고분자에 도입 CH2 CH O OCH2CH2OCCH BF3 etherate CH toluene 3 (9.38) CH2CH OCH2CH2OCCH CH ② 미리 형성된 고분자에 반응기를 첨가 O CH2CH + N CH2Cl SnCl4 CH2CH O O 4 CH2N O (9.39) 9.4.4 Crosslinking Through Labile Functional Groups Reaction between appropriate difunctional or polyfuntional reagents with labile groups on the polymer chains - Crosslinking H2N Ar NH 2 CH SO2NH Ar NHSO 2CH CH2 CH 2 CH2 (9.40) SO2Cl CH2 CH HO R OH SO2OROSO2 CH CH2 H2C (Friedel-Crafts reaction) CH 2 CH2 (9.41) + Cl R Cl SnCl 4 (9.42) CH CH2 R CH H 2C 9.4.4 Crosslinking Through Labile Functional Groups Cyclopentadiene-substituted polymer의 Diels-Alder reaction (9.43) + Thermoplastic Elastomers ( 열에 의해 재 가공이 가능) 9.4.5 Ionic Crosslinking The hydrolysis of chlorosulfonated polyethylene with aqueous lead oxide CH2CH PbO, H2O CH2CH CHCH2 SO2-Pb2+-O2S SO2Cl CH3 CH2CH2 5 CH2C CO2H poly[ethylene-co-(methacrylic acid)] 상품명 : ionomer (9.44) 9.4.5 Ionic Crosslinking Properties of Ionomers ① Introduction of ions causes disordering of the semicrystalline structure, which makes the polymer transparent. ② Crosslinking gives the polymer elastomeric properties, but it can still be molded at elevated temperatures. ③ Polarity , adhesion 9.4.5 Ionic Crosslinking Application of Ionomers coatings adhesive layers for bonding wood to metal blow-molded and injection-molded containers golf ball covers blister packaging material binder for aluminosilicate dental fillings 9.5 Block and Graft Copolymer Formation 9.5.1 Block Copolymers 1. Polymer containing functional end groups 사용 O CHCH2OH + OCN CHCH2 Ph Ph (9.45) OCNH 2. Peroxide groups introduced to polymer chain ends 사용 CH3 CH3 O2 CHCH2OC R CH3 CH3 CH CH3 CHCH2OC R (9.46) CH3 CH3 C OOH CH3 개시제의 역할 9.5 Block and Graft Copolymer Formation 9.5.1 Block Copolymers 3. Peroxide units 사용 2xCH2 CH + O2 initiator CH2CH X X CH2CH X O x O O CH2CH X x (9.47) x CHY + 2yCH2 CH2CH X O x (9.48) 2 CH2CH X O x O CH2CH X y 9.5 Block and Graft Copolymer Formation 9.5.1 Block Copolymers 4. Another way to form chain-end radicals - mechanical degradation of homopolymers (using ultrasonic radiation or high-speed stirring) EX) Polyethylene-block-polystyrene 9.5.2 Graft Copolymers A. Three general methods of preparing graft copolymers (1) A monomer is polymerized in the presence of a polymer with branching resulting from chain transfer. (2) A monomer is polymerized in the presence of a polymer having reactive functional groups or positions that are capable of being activated (3) Two polymers having reactive functional groups are coreacted. 9.5.2 Graft Copolymers (1) Chain transfer 1) Three components polymer, monomer, and initiator 2) The initiator may play one of two roles ① It polymerizes the monomer to form a polymeric radical (or ion or coordination complex), which, in reacts with the original polymer ② it reacts with the polymer to form a reactive site on the backbone which, in turn, polymerizes the monomer. 9.5.2 Graft Copolymers 3) Consideration ① reactivity ratios of monomers ② To take into account the frequency of transfer determine the number of grafts. 4) Grafting sites : That are susceptible to transfer reactions At carbons adjacent to double bonds in polydienes At carbons adjacent to carbonyl groups 9.5.2 Graft Copolymers At carbons adjacent to carbonyl groups EX . (CH2CH2)y CH2CH CH2CH OCCH3 CH 2 CH 2 CH2C OCCH2(CH2CH2).x (9.49) OCCH3 peroxide O O Mixture of poly(vinyl alcohol)-graft-polyethylene and long-chain carboxylic acids 5) Grafting efficiency improvement Group that undergoes radical transfer readily (mercaptan is incorporated into the polymer backbone. 9.5.2 Graft Copolymers 6) Cationic chain transfer grafting CH2CH+BF3OHPh CH2CH CH2CH + (9.50) HCH2C Friedel-Crafts attack OCH3 Ph OCH3 Styrene is polymerized with BF3 in the presence of poly(p-methoxystyrene) 9.5.2 Graft Copolymers (2) Grafting by activation by backbone functional groups CH2CH CH2CH CH2CH CH 2 Na-naphthalene CHCN (9.51) tetrahydrofuran Cl Na CH2CH CN Synthesis of poly(p-chlorostyrene)-graft-polyacrylonitrile 9.5.2 Graft Copolymers Irradiation – provide active sites with ultraviolet or visible radiation with or without added photosensitizer with ionizing radiation Major difficulty substantial amounts of homopolymerization = grafting settlement This has been obviated to some extent by preirradiating the polymer prior to addition of the new monomer. 9.5.2 Graft Copolymers Direct irradiation of monomer and polymer together Best combination Polymer – very sensitive to radiation Monomer – not very sensitive ( Sensitivity measurement – G values) TAB.9.2. Irradiation grafting of polymer emulsions - effective way to minimize 9.5.2 Graft Copolymers TABLE 9.2. Appoximate G Values of Monomers and Polymersa Monomer Butadiene G Very low Polymer Polybutadiene G 2.0 Styrene 0.70 Polystyrene 1.5-3 Ethylene 4.0 Polyethylene 6-8 Acrylonitrile 5.0-5.6 - Methyl methacrylate 5.5-11.5 Poly(methyl methacrylate) 6-12 Methyl acrylate 6.3 Poly(methyl acrylate) 6-12 Vinyl acetate 9.6-12.0 Poly(vinyl acetate) 6-12 Poly(vinyl chloride) 10-15 Vinyl chloride aData 10.0 - from Chapiro.72 G values refer to number of free radicals formed per 100 eV of energy absorbed per gram of material. Good Combination Poly(vinyl chloride) and butadiene 9.5.2 Graft Copolymers (3) Using two polymers O N + HO2C O CNHCH2CH2OC (9.52) O Grafting of an oxazoline-substituted polymer with a carboxyl-terminated polymer 9.6 Polymer Degradation 9.6.1 Chemical Degradation Breakdown of the polymer backbone No involving pendant groups. No functional groups other than Limit to oxidation ( with oxygen) 9.6 Polymer Degradation 9.6.1 Chemical Degradation Saturated polymers Very slowly by oxygen Autocatalytic Speed up – heat or light or by presence of certain impurities Reaction Product – numerous and include water, carbon dioxide, carbon monoxide, hydrogen, and alcohols Tertiary carbon atoms – most susceptible to attack (polyisobutylene>polyethylene>polypropylene) Crosslinking – always degradation Decomposition of initially formed hydroperoxide groups - mainly responsible for chain scission OOH O CH2CHCH2CCH2CH R R R CH2CHCH2 + CCH2CH R R + OH R Decomposition of initially formed hydroperoxide groups (9.53) 9.6 Polymer Degradation 9.6.1 Chemical Degradation Unsaturated polymers Much more rapidly (by complex free radical ) Allylic carbon atoms – most sensitive to attack (resonance-stabilized radicals) very susceptible to attack by ozone 9.6.2 Thermal Degradation Three types of thermal degradation (1) nonchain scission (2) random chain scission (3) depropagation (1) nonchain scission ① refers to reactions involving pendant groups CH CH2CH 2CH CH OCCH + HOCCH OCCH333 CH CH CH CH OO O HOCCH3 ++ HOCCH 3 O O (9.54) elimination of acid from poly(vinyl esters) CH2CH O C OCH2CH2R CH2CH O + CH2 CHR C OH elimination of alkene from poly(alkyl acrylate)s (9.55) 9.6.2 Thermal Degradation ② Use – approach to solving the problems of polyacetylene’s intractability CF3 CF3 CF3 WCl6 CF3 (9.56) Sn(CH3)4 7 tricyclic monomer 8 precursor polymer Thermal degradation 8 Durham route CF3 + (9.57) CF3 coherent films of polyacetylene Disadvantage of the Durham route : That a relatively large molecule needs to be eliminated. Yield polyacetylene without the necessity of an elimination reaction CH CH Hg2+ CH CH CH CH CH CH (9.58) 9.6.2 Thermal Degradation ③ Useful of Nonchain scission reactions : Characterizing copolymers (when the amount of a volatile degradation product can be correlated with the concentration of a given repeating unit) (2) Random chain scission : Result from homolytic bond-cleavage reactions at weak points in the polymer chains CH2CH2CH2CH2 CH2CH2 + CH2CH2 CH CH2 + CH3CH2 (9.59) 9.6.2 Thermal Degradation (3) Depropagation Initiation Chain end Poly(methyl methacrylate) Random site along the backbone Poly(-methylstyrene) In both case R R R CH2CCH2C CH2C R R R R + CH2 C R (9.60) 9.6.3 Degradation by Radiation Radiation crosslinking or degradation Ultraviolet or visible light Elevated temperatures - 1,1-disubstituted polymers to degrade to monomer Room temperature – crosslinking and chain scission reactions Ionizing radiation much higher yields of monomer from 1,1-disubstituted polymers at room temperature. At comparable levels of radiation polyethylene and monosubstituted polymers – mainly crosslinking All vinyl polymers – tend to degrade – under very high dosages of radiation. 9.6.3 Degradation by Radiation SCHEME 9.3. Random chain scission of polyethylene.