Solution - Fccj.us
advertisement

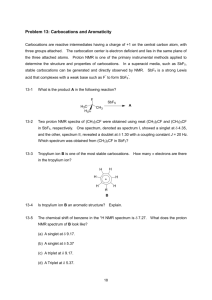
CONCEPTUAL EXAMPLE 10.1 Repeat Units in Polymers What is the repeat unit in polyvinylidene chloride? A segment of the polymer is represented as H Cl H Cl H Cl ~C C C C C C~ Cl H Cl H Cl H Solution The repeat unit is H Cl C C H Cl Joining three of these units forms the segment shown; joining hundreds of the units would form a molecule of the polymer. Exercise 10.1 What is the repeat unit in polyacrylonitrile? A segment of the polymer is represented as ~CH2CHCH2CHCH2CHCH2CHCH2CHCH2CH~ CN CN CN CN CN CN EXAMPLE 10.2 Structures of Polymers Give the structure of the polymer made from vinyl fluoride (CH2 CHF). Show at least four repeat units. Solution The carbon atoms become bonded in a chain with only single bonds between the carbon atoms. The fluorine atom is a substituent on the chain. (Two of the electrons in the double bond of the monomer are used to join the units.) The polymer is ~CH2CHCH2CHCH2CHCH2CH~ F F F F Exercise 10.2A Give the structure of the polymer made from methyl vinyl ether (CH 2 repeat units. CHOCH3). Show at least four Exercise 10.2B Give the structure of the polymer made from vinyl acetate (CH2 repeat units. CHOCOCH3). Show at least four EXAMPLE 10.3 Condensed Structural Formulas for Polymers Write the condensed structural formula for the polymer formed from dimethylsilanol, (CH 3)2Si2(OH)2. Solution Let’s start by writing out the structural formula of the monomer. CH3 HO Si OH CH3 Because there are no double bonds in this molecule, we do not expect addition polymerization. Rather, we expect a condensation reaction in which an OH group of one molecule and an H atom of another combine to form a molecule of water. Moreover, because there are two OH groups per molecule, each monomer can form bonds with the neighbors on both sides. This is a key requirement for polymerization. We can represent the reaction as follows. CH3 HO Si CH3 CH3 OH + HO Si CH3 CH3 OH + HO Si CH3 CH3 OH + • • • Si CH3 O + n H2O n EXAMPLE 10.03 Condensed Structural Formulas for Polymers continued Exercise 10.3 (a) Write the structural formula for the polymer formed from 3-hydroxypropanoic acid (HOCH2CH2COOH), showing at least four repeat units. (b) Write a condensed structural formula in which the repeat unit is shown in brackets.