General Approaches to Polymer Synthesis
advertisement

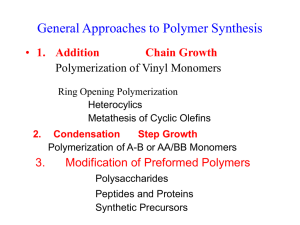
General Approaches to Polymer Synthesis 1. Addition Chain Growth Polymerization of Vinyl Monomers 2. Ring Opening Polymerization Heterocylics Methathesis of Cyclic olefins 3. Condensation Step Growth Polymerization of A-B or AA/BB Monomers 4. Modification of Preformed Polymers Polysaccharides Peptides and Proteins Synthetic Precursors Current Strategies in Polymer Synthesis Objectives: Precise Macromolecular Design 1. Control of: Molecular Weight Composition Sequence of repeat units Stereochemistry 2. Versatility Synthetic Methods 1. Step-growth (Condensation) Polymerization Mw/Mn 2 Statistical compositions and sequences 2. Chain-growth (Addition) Polymerization Mw/Mn > 1.5 2.0 Statistical compositions and sequences Little stereochemical control Major Developments in the 1950-60's 3. Living Polymerization (Anionic) Mw/Mn 1 Blocks, telechelics and stars available (Controlled molecular architecture) Statistical Stereochemical Control Statistical Compositions and Sequences Severe functional group restrictions 4. Ziegler-Natta (Metal-Coordinated) Polymerization Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i.e. olefins Additional Developments in the 1980's 5. "Immortal" Polymerization (Cationic) Mw/Mn 1.05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Severe functional group restrictions Under Development in the 1990's 6. "Living" Free Radical Polymerization Mw/Mn 1.05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Broad range of monomers available 7. Genetic Approaches via Modified Microorganisms Monodisperse in MW Monodisperse in Composition Sequentially Uniform Stereochemically Pure Diverse set of functional groups possible Structural Complexity of Polymers Homopolymers Head to Tail vs. Head to Head Adducts 1,2- vs 1,4 Adducts Tacticity of Enchainments Branching Copolymers Identity and Number of Comonomers Ratio and Distribution of Comonomers Statistical Alternating Blocks Grafts Molecular Weight Mn, Mw, Mz, Mv Averages Molecular Weight Distribution Crosslinking Density Length of Crosslinks Morphology Impurities Additives Structural Complexity of Polymers Time Dependent Changes Chemical Reactions Hydrolysis Dehydrohalogenation Photodegradation Oxidation Thermal Degradation Processing Aging Crystallization Changes in Polymorphism Weathering Plasticizer Loss -- Imbrittlement Types of Intermolecular Forces Type of Force Relative Strength Low Molecular Analog Polymer Dispersion or Van der Waals Weak Methane Hexane Polyethylene Polypropylene DipoleDipole Medium CH3Cl PVC CH3CO2CH3 PMMA Hydrogen bonding Strong H2O CH3CONH2 Proteins Cellulose Polyamide Electrostatic Very Strong NaCl Ionomers + or Ionic CH3CO2 Na Vinyl Monomers CH2=CH X X H Polymer Polyethylene Abbreviation PE CH3 Polypropylene PP Cl Poly(vinyl chloride) PVC Phenyl Polystyrene PSt CN Polyacrylonitrile PAN COOCH3 Poly(methyl acrylate) PMA O-COCH3 Poly(vinyl acetate) PVAc Vinylidene Monomers X CH2=C Y X CH3 Y CH3 Polymer Polyisobutylene Abbreviation PIB Cl Cl Poly(vinylidene chloride) PVDC F F Poly(vinylidene fluoride) PVDF Phenyl CH3 Poly(-methyl styrene CH3 COOCH3 Poly(methyl methacrylate) CN COOR Poly(alkyl cyanoacrylate PMMA Tacticity Isotactic H H H X X X X X X All asymmetric carbons have same configuration Methylene hydrogens are meso Polymer forms helix to minimize substituent interaction Syndiotactic H X X X X X X Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation Heterotactic (Atactic) H XX X X X X Asymmetric carbons have alternate configuration