Training
advertisement

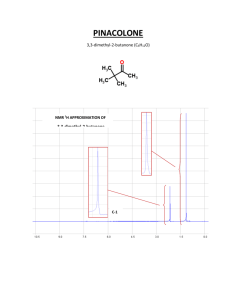
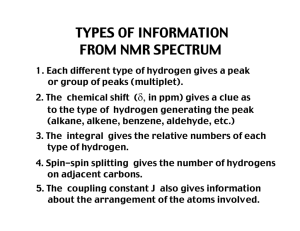
Chapter 13 Nuclear Magnetic Resonance Spectroscopy Assignment for Chapter 13 • 13.1 through 13.14 13.16 and 13.18 • SKIP 13.15 and 13.17 Problem Assignment • In text problems 2 - 16 22 a, b, d, g 24, 25 • End of chapter problems 27, 28, 29, 30, 31, 35, 39a, b 40 a, c, d, f 41 a, b, c, d 13.1 Spin Quantum Numbers of Some Nuclei The most abundant isotopes of C and O do not have spin. Element Nuclear Spin Quantum No 1H 2H 12C 13C 14N 16O 17O 19F 1/2 1 0 1/2 1 0 5/2 1/2 2 3 0 2 3 0 6 2 (I) No. of Spin States Elements with either odd mass or odd atomic number have the property of nuclear “spin”. The number of spin states is 2I + 1, where I is the spin quantum number. 13.2 Nuclear Resonance absorption of energy by the spinning nucleus Nuclear Spin Energy Levels N -1/2 unaligned In a strong magnetic field (Bo) the two spin states differ in energy. +1/2 Bo S aligned The Energy Separation Depends on Bo - 1/2 DE = kBo = hn degenerate at Bo = 0 + 1/2 Bo increasing magnetic field strength The Larmor Equation!!! DE = kBo = hn can be transformed into gyromagnetic frequency of the incoming radiation that will cause a transition nn == gBg0 2p 2p ratio g Bo strength of the magnetic field g is a constant which is different for each atomic nucleus (H, C, N, etc) Resonance Frequencies of Selected Nuclei Isotope Abundance Bo (Tesla) 1H 13C Frequency(MHz) g(radians/Tesla) 99.98% 1.00 1.41 2.35 7.05 42.6 60.0 100.0 300.0 267.53 1.108% 1.00 2.35 7.05 10.7 25.0 75.0 67.28 13.3 Classical Instrumentation: The Continuous-Wave NMR typical before 1965; field is scanned A Simplified 60 MHz NMR Spectrometer RF (60 MHz) Oscillator hn Transmitter absorption signal RF Detector Recorder Receiver MAGNET MAGNET N S Probe ~ 1.41 Tesla (+/-) a few ppm NMR Spectrum of Phenylacetone O CH2 C CH3 EACH DIFFERENT TYPE OF PROTON COMES AT A DIFFERENT PLACE - YOU CAN TELL HOW MANY DIFFERENT TYPES OF PROTONS THERE ARE BY INTEGRATION. 13.4 Modern Instrumentation: the Fourier-Transform NMR FT-NMR requires a computer PULSED EXCITATION N n1 BROADBAND RF PULSE contains a range of frequencies (n1 ..... nn) n2 O CH2 C CH3 n3 S All types of hydrogen are excited simultaneously with the single RF pulse. 13.5 Proton NMR Spectrum NMR Spectrum of Phenylacetone O CH2 C CH3 EACH DIFFERENT TYPE OF PROTON COMES AT A DIFFERENT PLACE - YOU CAN TELL HOW MANY DIFFERENT TYPES OF PROTONS THERE ARE BY INTEGRATION. Some Generalizations • • • • NMR solvents contain deuterium Tetramethylsilane (TMS) is the reference Spectrum of 1,2,2-trichloropropane Chemical shift in Hz from TMS vary according to frequency of spectrometer! • Delta values (d) are independent of frequency of spectrometer (ppm) PEAKS ARE MEASURED RELATIVE TO TMS Rather than measure the exact resonance position of a peak, we measure how far downfield it is shifted from TMS. reference compound tetramethylsilane “TMS” CH3 CH3 Si CH3 CH3 Highly shielded protons appear way upfield. TMS shift in Hz downfield n 0 Chemists originally thought no other compound would come at a higher field than TMS. HIGHER FREQUENCIES GIVE LARGER SHIFTS The shift observed for a given proton in Hz also depends on the frequency of the instrument used. Higher frequencies = larger shifts in Hz. TMS shift in Hz downfield n 0 THE CHEMICAL SHIFT The shifts from TMS in Hz are bigger in higher field instruments (300 MHz, 500 MHz) than they are in the lower field instruments (100 MHz, 60 MHz). We can adjust the shift to a field-independent value, the “chemical shift” in the following way: parts per million chemical = shift d shift in Hz = ppm = spectrometer frequency in MHz This division gives a number independent of the instrument used. A particular proton in a given molecule will always come at the same chemical shift (constant value). 13.6 and 13.7 The Chemical Shift Generalizations • electrons shield nucleus • electronegativity: withdraws electrons to deshield nucleus • downfield (deshielding) = left side of spectrum • upfield (shielding) = right side of spectrum • delta values increase from right to left! PROTONS DIFFER IN THEIR SHIELDING All different types of protons in a molecule have a different amounts of shielding. They all respond differently to the applied magnetic field and appear at different places in the spectrum. This is why an NMR spectrum contains useful information (different types of protons appear in predictable places). DOWNFIELD Less shielded protons appear here. SPECTRUM UPFIELD Highly shielded protons appear here. It takes a higher field to cause resonance. Overview of where protons appear in an NMR spectrum C-H where C is CH on C attached to an aliphatic acid aldehyde benzene alkene next to C-H COOH CHO CH =C-H electronega- pi bonds tive atom X=C-C-H X-C-H 12 10 9 7 6 4 3 2 0 NMR Correlation Chart -OH -NH DOWNFIELD DESHIELDED UPFIELD SHIELDED CHCl3 , H TMS 12 11 10 9 8 7 6 H RCOOH RCHO C=C 5 4 CH2F CH2Cl CH2Br CH2I CH2O CH2NO2 3 2 1 0 CH2Ar C-CH-C CH2NR2 C CH2S C-CH2-C C C-H C=C-CH2 C-CH3 CH2-CO d (ppm) Some approximate NMR values (part 1) H R C O 7-8 ppm 11-12 ppm H-O carboxylic acid benzene ring protons R C H aldehyde O 9-10 ppm C 5-7 ppm C H vinyl protons (alkene) Some approximate NMR values (part 2) -CH2-O- about 4ppm -CH2-X about 3.5 ppm -CH2-CH2-O- about 1.2 ppm for boldfaced CH2 X = Cl, Br, I If two chlorine atoms are attached, shifts to 5.3 ppm CH3 CH2 CH about 1 to 1.5 ppm O and CH 2 long way from electronegative atoms R about 2 ppm CH 2- DESHIELDING AND ANISOTROPY Three major factors account for the resonance positions (on the ppm scale) of most protons. 1. Deshielding by electronegative elements. 2. Anisotropic fields usually due to pi-bonded electrons in the molecule. 3. Deshielding due to hydrogen bonding. DESHIELDING BY ELECTRONEGATIVE ELEMENTS DESHIELDING BY AN ELECTRONEGATIVE ELEMENT d- Cl d+ C d- electronegative element H d+ Chlorine “deshields” the proton, that is, it takes valence electron density away from carbon, which in turn takes more density from hydrogen deshielding the proton. NMR CHART “deshielded“ protons appear at low field highly shielded protons appear at high field deshielding moves proton resonance to lower field Electronegativity Dependence of Chemical Shift Dependence of the Chemical Shift of CH3X on the Element X Compound CH3X Element X Electronegativity of X Chemical shift d most deshielded CH3F CH3OH CH3Cl CH3Br CH3I CH4 (CH3)4Si F O Cl Br I H Si 4.0 3.5 3.1 2.8 2.5 2.1 1.8 4.26 3.40 3.05 2.68 2.16 0.23 0 TMS deshielding increases with the electronegativity of atom X Substitution Effects on Chemical Shift most deshielded most deshielded CHCl3 CH2Cl2 CH3Cl 7.27 5.30 3.05 ppm -CH2-Br 3.30 -CH2-CH2Br 1.69 The effect increases with greater numbers of electronegative atoms. -CH2-CH2CH2Br 1.25 ppm The effect decreases with incresing distance. ANISOTROPIC FIELDS DUE TO THE PRESENCE OF PI BONDS The presence of a nearby p bond greatly affects the chemical shift. Benzene rings have the greatest effect. Ring Current in Benzene Circulating p electrons H Bo H Deshielded fields add together Secondary magnetic field generated by circulating p electrons deshields aromatic protons ANISOTROPIC FIELD IN AN ALKENE protons are deshielded Deshielded fields add H shifted downfield C=C H Bo H H secondary magnetic (anisotropic) field lines ANISOTROPIC FIELD FOR AN ALKYNE H C C H Bo Shielded fields subtract hydrogens are shielded secondary magnetic (anisotropic) field HYDROGEN BONDING HYDROGEN BONDING DESHIELDS PROTONS R O H H O H O R The chemical shift depends on how much hydrogen bonding is taking place. Alcohols vary in chemical shift from 0.5 ppm (free OH) to about 5.0 ppm (lots of H bonding). R Hydrogen bonding lengthens the O-H bond and reduces the valence electron density around the proton - it is deshielded and shifted downfield in the NMR spectrum. SOME MORE EXTREME EXAMPLES O H O C R R C O H O Carboxylic acids have strong hydrogen bonding - they form dimers. With carboxylic acids the O-H absorptions are found between 10 and 12 ppm very far downfield. H3C O O H O In methyl salicylate, which has strong internal hydrogen bonding, the NMR absortion for O-H is at about 14 ppm, way, way downfield. Notice that a 6-membered ring is formed. 13.8 Integration INTEGRATION OF A PEAK Not only does each different type of hydrogen give a distinct peak in the NMR spectrum, but we can also tell the relative numbers of each type of hydrogen by a process called integration. Integration = determination of the area under a peak The area under a peak is proportional to the number of protons that generate the peak. Benzyl Acetate The integral line rises an amount proportional to the number of H in each peak METHOD 1 integral line integral line 55 : 22 : 33 = 5:2:3 simplest ratio of the heights Benzyl Acetate (FT-NMR) Actually : 5 58.117 / 11.3 = 5.14 2 21.215 / 11.3 = 1.90 3 33.929 / 11.3 = 3.00 O CH2 O C CH3 METHOD 2 digital integration assume CH3 33.929 / 3 = 11.3 per H Integrals are good to about 10% accuracy. Modern instruments report the integral as a number.