The Mass Spectrometer
advertisement

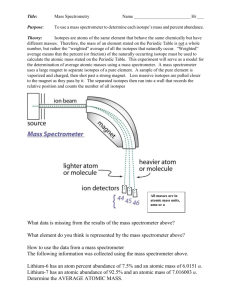
The Mass Spectrometer Topic 2.2 Review of Topic 2.1 Mass Number (A) protons + neutrons A Z X n+/n- Atomic Number (Z) number of protons The symbol (X) for a given element Charge (n) Atoms have no charge, so this is left blank. Ions are atoms that have gained or lost electrons, the charge is indicated here. Mass Spectrometer (Topic 2.2) • has many applications, but one of the simplest is to determine the natural abundances of the isotopes of a particular element – the relative atomic mass can be calculated from the data from the mass spectrometer Mass spectrometer video (2:26) http://www.youtube.com/watch?v=_L4U6ImYSj0 • a positively charged particle is deflected along a circular path that is proportional to its mass/charge ratio – m/z • mass is m • charge is z • occurs in a vacuum • the machine can be adjusted in order to look at certain particles • Five parts (a simple diagram with these parts is required) – vaporization • a substance is first converted to a vapor/gaseous state – ionization • the sample (atoms or molecules) are bombarded with a stream of electrons – the collisions knock one or more electrons off to make positive ions – normally one electron is knocked off leaving a 1+ charge –acceleration • sample is accelerated through a magnetic field –deflection • ions are deflected with a magnet and electromagnetic field • deflections depends on: – lighter particles deflect more – higher positive charged particles deflect more –detection • particles with different masses will be detected at different points at the end deflection Carbon- 12 as a standard • carbon- 12 – ALL masses on the periodic table are based on their relationship to carbon-12 • the C-12 atom has been given the atomic weight of exactly 12.000000000 and is used as the basis upon which the atomic weight of other isotopes is determined Calculate the relative atomic mass of magnesium with the provided data. (2.2.3) • magnesium results from the mass spectrometer: – 80% – 10% – 10% 24Mg 25Mg 26Mg • just a simple weighted mean – .80(24) + .10(25) + .10(26) = 24.3 amu Calculate the abundance (the % of each isotope found in nature) of each isotope (2.2.3) • Rubidium (Rb) has relative atomic mass of 85.47 and two isotopes – rubidium- 85 and rubidium- 87 • make rubidium 85 = x • make rubidium 87 = y – (x · 85) + (y · 87) = 85.47 • x+y=1 • therefore substitute (1 – x) for y Be clear with your answer and state the percent of each isotope. – (x · 85) + ((1-x) · 87) = 85.47 • solve for x • x = .765 or 76.5% for rubidium- 85 • therefore y = .235 or 23.5% for rubidium- 87