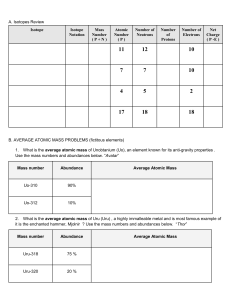
Mass Spectrometry Do now: Calculate the relative formula masses for: (without looking back at your notes) Zinc chloride, ZnCl2 Sulphuric acid, H2SO4 Ethanol, C2H4(OH)2 CH3COONa C(CH3)4 Calculating average atomic mass Calculating average atomic mass How do you calculate it? 6.92amu 10.80amu 24.32amu 28.10amu 52.05amu Mass Spectrometer Draw and annotate what is happening on each part of the mass spectrometer as you watch the video. Mass Spectrometer What does a mass spectra look like? REMINDER - How do you calculate it? Element Chlorine Relative mass of isotope 35 37 Relative abundance 3 1 1. Multiply the mass of each isotope by its relative abundance 2. Add those together 3. Divide by the sum of the relative abundances R.A.M. = (35 x 3) + (37 x 1) 3+1 = 35.5 Calculate the RAM of the different isotopes. What is the element? A B D C E ANSWERS ANSWERS Exam question Exam question Self assessment Self assessment