Mass Spec and Average Atomic Mass
advertisement
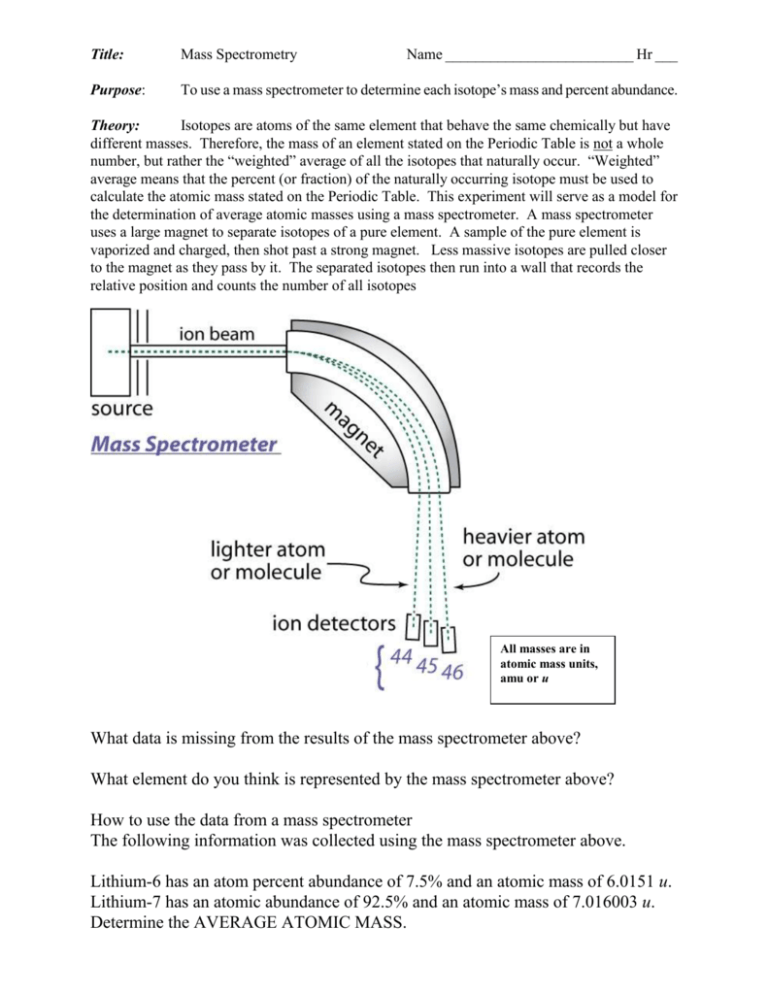
Title: Mass Spectrometry Name _________________________ Hr ___ Purpose: To use a mass spectrometer to determine each isotope’s mass and percent abundance. Theory: Isotopes are atoms of the same element that behave the same chemically but have different masses. Therefore, the mass of an element stated on the Periodic Table is not a whole number, but rather the “weighted” average of all the isotopes that naturally occur. “Weighted” average means that the percent (or fraction) of the naturally occurring isotope must be used to calculate the atomic mass stated on the Periodic Table. This experiment will serve as a model for the determination of average atomic masses using a mass spectrometer. A mass spectrometer uses a large magnet to separate isotopes of a pure element. A sample of the pure element is vaporized and charged, then shot past a strong magnet. Less massive isotopes are pulled closer to the magnet as they pass by it. The separated isotopes then run into a wall that records the relative position and counts the number of all isotopes All masses are in atomic mass units, amu or u What data is missing from the results of the mass spectrometer above? What element do you think is represented by the mass spectrometer above? How to use the data from a mass spectrometer The following information was collected using the mass spectrometer above. Lithium-6 has an atom percent abundance of 7.5% and an atomic mass of 6.0151 u. Lithium-7 has an atomic abundance of 92.5% and an atomic mass of 7.016003 u. Determine the AVERAGE ATOMIC MASS. Procedures: 1. 2. Each of the following elements were purified and analyzed using a mass spectrometer. For each of the following elements determine the average atomic mass. SHOW YOUR WORK. Data: Potassium Nuclide K-39 K-40 K-41 Boron Nuclide B-10 B-11 Abundance 93.22% 0.118% 6.77% Abundance 19.7% 80.3% Mass 39.0 40.0 41.0 Mass 10.01 11.01 Magnesium Nuclide Mg-26 Mg-24 Mg-25 Abundance 11.17% 78.7% 10.13% Mass 26.0 24.0 25.0 Copper Nuclide Cu-63 Cu-65 Abundance 69.09% 30.91% Mass 62.9 64.9 Which isotope is most abundant? There are three isotopes of Hydrogen, H-1, H-2, and H-3. The average atomic mass of Hydrogen is 1.01 u. There are two isotopes of Chlorine, Cl-35 and Cl-37. The average atomic mass of chlorine is 35.45u. There are five isotopes of Nickel, Ni-58, Ni-60, Ni-61, Ni-62, Ni-64. The average atomic mass of Nickel is 58.71 u.









