Lehninger Principles of Biochemistry
advertisement
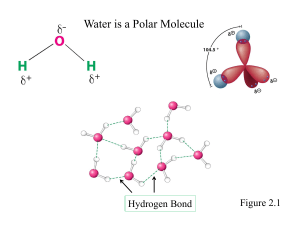
David L. Nelson and Michael M. Cox LEHNINGER PRINCIPLES OF BIOCHEMISTRY Sixth Edition CHAPTER 1 The Foundations of Biochemistry © 2013 W. H. Freeman and Company Pancreatic cell section Universal features of living cells Phylogeny of the three domains of life Classification of Organisms Several functional groups in a single biomolecule Complementary fit between a macromolecule and a small molecule Stereoisomers have different effects in humans Many drugs are racemic mixtures Energy Transformations in Living Organisms The major carrier of chemical energy in all cells is adenosine triphosphate (ATP) David L. Nelson and Michael M. Cox LEHNINGER PRINCIPLES OF BIOCHEMISTRY Sixth Edition CHAPTER 2 Water © 2013 W. H. Freeman and Company Water Hydrogen bonding in ice Common hydrogen bonds in biological systems Biologically important hydrogen bonds Directionality of the hydrogen bond Water as a solvent Amphipathic compounds in aqueous solution Dispersion of lipids in water Release of ordered water is energetically favorable Ionization of water Conjugate acid-base pairs consist of a proton donor and a proton acceptor Titration curve of acetic acid H2O H2O Equilibrium = Keq = constant Ion product of water = OH- + H+ [OH-] [H+] = 1.8 x 10-16 M [H2O] Kw = [OH-] [H+] = 10-14 M2 Pure H2O : [H+] = [OH-] = 10-7 M pH = - log [H+] = -log (10-7) = 7 If [H+] > 10-7 M then pH < 7 (acidic) If [H+] < 10-7 M then pH > 7 (basic) Blood: [H+] = 4 x 10-8 M Blood pH = 7.4 Acids and Bases Acid (HA) - something that has a proton and is willing to give it up. Base (A-) - something that has a place to put a proton HA H + + A- HA + H2O H 3O+ + A- Strong acids completely dissolve in H2O (H+Cl-). Weak acids don’t completely dissolve in H2O. [A-] [H+] K= O [HA] H2PO4(acid) O HPO42- + H+ (base) P OH Ka = [HPO42-] [H+] [H2PO4-] pKa = - log (Ka) = 6.82 = 1.51 x 10-7 M OH Relationship between pH and pKa Henderson – Hasselbalch equation pH = pKa when: The molar concentration of acid and conjugate base are equal [H2PO4-] = [HPO42-] pH = pKa = 6.8 Physiological pH The pH in the human body needs to remain ~7. Enzyme catalysis, protein-protein interactions, receptor binding, and other biological processes are very sensitive to pH. pH balance of the blood is maintained using a CO2 - bicarbonate buffer. CO2 + H2O (acid) H2CO3 (hydrated CO2) H+ + HCO3- (bicarbonate base) pKa = 6.1 There is more than 10-fold more base (HCO3-) than acid (CO2) so pH > pK (pH= 7.4) CO2 is exhaled by the lungs H+ + HCO3- CO2 + H2O Breathing rate controls CO2 CO2 balance is controlled by the lungs, HCO3- by the kidneys