Valence Bond Theory
advertisement

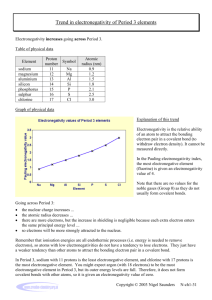
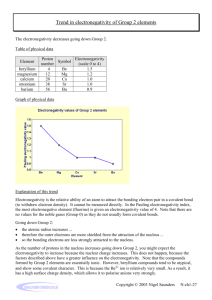
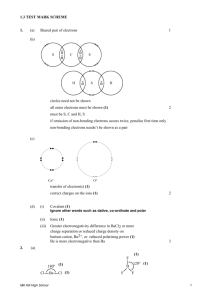
Valence Bond Theory & Hybridization p. 224-235 1. The salt compound, potassium bromide, possesses anticonvulsant properties but is presently used by veterinarians for treating epilepsy in animals. a. Identify the type of bonding in potassium bromide. Provide at least two reasons for the type of bonding involved in potassium bromide. Ionic bonding. Transfer of electrons from a metal to a nonmetal. Electronegativity difference > 1.67 b. Which element has the larger atomic radius: potassium or bromine? Explain. Potassium, atomic radius decreases across a group due to increased electron shielding. c. Use Lewis structures to show the formation of potassium bromide. d. Use orbital diagrams to show the formation of potassium bromide. e. Which orbitals are involved in the reaction between potassium and bromine? 4s and 4p f. Describe what happens to the size of potassium and bromine atoms when they become ions. Potassium decreases in size because it loses an electron from the 4s orbital. Bromine gains an electron in the 4p orbital so it increases in size. g. Provide three physical properties conferred by the type of bonding in potassium bromide. Crystalline structure, brittle, high melting & boiling point, solid at SATP, electrolyte in aqueous solution, high solubility in water (some exceptions). 2. Hydrochloric acid, HCl(aq), is a strong acid commonly used in high school laboratories. a. Determine the difference in electronegativity between hydrogen and chlorine and identify the type of bonding involved. Provide reasoning. Polar covalent. Electronegativity difference of 0.9, unequal sharing of electrons between two nonmetals. b. Use Lewis structures to show the formation of hydrochloric acid. c. Use orbital diagrams to show the formation of hydrochloric acid. 3. Water, H2O(l) is considered the universal solvent. Answer question 2 for water. Polar covalent. Electronegativity difference of 1.4, unequal sharing of electrons between two nonmetals. 4. Beryllium hydride, BeH2(s), is a solid compound that does not exhibit the physical properties of ionic compounds, such as its low melting point. a. Determine the difference in electronegativity between beryllium and hydrogen and identify the type of bonding involved. Electronegativity difference of 0.6, polar covalent bond NOT ionic. b. Draw the energy level diagrams for beryllium and hydrogen. What orbitals will form covalent bonds? c. Explain how beryllium will form two equivalent covalent bonds to form beryllium hydride. Hybridization (combination of atomic orbitals to create a new orbital). One electron from the sorbital is promoted and one electron from the p-orbital is demoted to form 2 equivalent sp hybrid orbitals. 5. Borane, BH3(g), is only found in the gaseous state indicating that it is a molecular compound. The three B-H bonds formed in borane are all equivalent covalent bonds, equal strengths and lengths. a. Draw the Lewis structure of borane. Explain why this is an example of an “incomplete octet”. Only 6 electrons. b. In which orbitals does boron have valence electrons? 2s and 2p orbitals c. Describe how boron must arrange its valence electrons to form three equal bonds. sp2 hybridization. 6. Methane, CH4(g), is the simplest hydrocarbon, a group of molecular compounds composed of carbon and hydrogen which form very strong covalent bonds. a. Draw the Lewis structure of methane. b. What is the electron configuration of the carbon atom? 1s22s22p2 c. Describe how carbon must arrange its valence electrons to form four equal bonds.