Sheet 4.2 Phases change - Science for the NZ Curriculum
advertisement

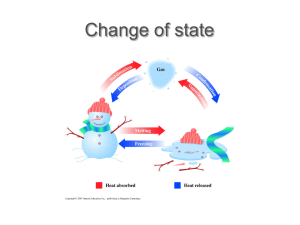
Sheet 4.2 Revision Phase change Changes between phases have special names. At each phase change energy is either transferred into or out of the substance. When a solid is given enough energy to break the bonds between each particle melting occurs. As the particles in a liquid gain energy they may be able to escape the liquid altogether and become a gas. This is evaporation. Boiling occurs when there is enough energy available for evaporation to occur inside a liquid; bubbles are formed. Under the right conditions, a solid can change directly into a gas. This is called sublimation. Dry ice (carbon dioxide) and iodine are solids that can undergo sublimation at room temperature. When gas particles lose enough energy to undergo a phase change, the gas becomes a liquid. This is called condensation. For example, as water vapour cools it condenses into liquid water. This is what causes the windows inside a building to fog up when it is cold outside. Heat energy from the water vapour inside a room is transferred through the glass to the cold air outside, turning the vapour into a liquid. When enough heat energy is lost from a liquid it freezes into a solid. Under the right conditions, a gas is able to change directly into a solid. This is called deposition. Frost is an example of deposition – it occurs when water vapour in the air has turned directly into solid ice. phase change The transition from one phase (solid, liquid or gas) to another melting The phase change where a solid becomes a liquid evaporation The phase change where a liquid becomes a gas boiling The phase change where a liquid becomes a gas, due to evaporation occurring inside the liquid sublimation The phase change where a solid becomes a gas condensation The phase change where a gas becomes a liquid vapour A gas that is at a low enough temperature that it can be condensed by pressure alone freezing The phase change where a liquid becomes a solid deposition The phase change where a gas becomes a solid Science for the New Zealand Curriculum Years 9 and 10 © Donald Reid, Catherine J. Bradley, Des Duthie, Catherine Low, Matthew McLeod, Colin Price 2010 Published by Cambridge University Press www.nzscience.co.nz www.cambridge.edu.au